The general soil contamination is characterized by the gross amount of heavy metal. The availability of elements for plants is determined by their mobile forms. Therefore, the content of mobile forms of heavy metals in the soil is the most important indicator characterizing the sanitary and hygienic situation and determining the need for ameliorative detoxification measures.
Depending on the extractant used, different amounts of the mobile form of the heavy metal are extracted, which with a certain convention can be considered accessible to plants. To extract mobile forms of heavy metals, various chemical compounds with different extracting powers are used: acids, salts, buffer solutions and water. The most common extractants are 1N HCl and ammonium acetate buffer with pH 4.8. At present, insufficient experimental material has yet been accumulated to characterize the dependence of the content of heavy metals in plants, extracted with various chemical solutions, on their concentration in the soil. The complexity of this situation is also due to the fact that the availability of the mobile form of heavy metal for plants depends largely on the properties of the soil and the specific characteristics of the plants. Moreover, the behavior of each element in the soil has its own specific, inherent patterns.
To study the influence of soil properties on the transformation of heavy metal compounds, model experiments were carried out with soils that sharply differ in properties (Table 8). Strong acid 1N HNO3, neutral salt Ca(NO3)2, ammonium acetate buffer solution and water were used as extractants.

The analytical data presented in tables 9-12 indicate that. that the content of acid-soluble compounds of zinc, lead and cadmium, passing into the 1N HNO3 extract, is close to their amount added to the soil. This extractant extracted 78-90% Pb, 88-100% Cd and 78-96% Zn entered the soil . The number of firmly fixed compounds of these elements depended on the level of soil fertility. Their content in poorly cultivated soddy-podzolic soil was lower than in moderately cultivated soddy-podzolic and typical chernozem.
The amount of exchangeable compounds Cd, Pb and Zn extracted by a 1-N solution of neutral salt Ca(NO3)2 was several times less than their mass added to the soil and also depended on the level of soil fertility. Lowest content elements extracted by Ca(NO3)2 solution were obtained on chernozem. With the increasing cultivation of soddy-podzolic soil, the mobility of heavy metals also decreased. Judging by the salt extract, cadmium compounds are the most mobile, and zinc compounds are somewhat less mobile. Lead compounds extracted with neutral salt were characterized by the lowest mobility.
The content of mobile forms of metals extracted by ammonium acetate buffer solution with pH 4.8 was also determined primarily by the type of soil, its composition and physicochemical properties.
As with the exchangeable (extractable by 1 N Ca(NO3)2) forms of these elements, the pattern remains, expressed in an increase in the number of mobile compounds of Cd, Pb and Zn in acidic soil, and the mobility of Cd and Zn is higher than that of Pb. The amount of cadmium extracted by this extract was 90-96% of the applied dose for poorly cultivated soil, 70-76% for medium-cultivated soddy-podzolic soil, and 44-48% for chernozem. The amount of zinc and lead passing into the CH3COONH4 buffer solution is equal, respectively: 57-71 and 42-67% for sod-podzolic poorly cultivated soil, 49-70 and 37-48% for moderately cultivated soil; 46-65 and 20-42% for black soil. The decrease in the extraction capacity of CH3COONH4 for lead on chernozem can be explained by the formation of more stable complexes and compounds with stable humus compounds.
The soils used in the model experiment differed in many respects soil fertility, but to the greatest extent in terms of acidic characteristics and the number of exchangeable bases. The experimental data available in the literature and the experimental data we obtained indicate that the reaction of the environment in the soil greatly affects the mobility of elements.
An increase in the concentration of hydrogen ions in the soil solution led to the transition of slightly soluble lead salts into more soluble salts (the transition of PbCO3 to Pb(HCO3)2 is especially characteristic (B.V. Nekrasov, 1974). In addition, with acidification, the stability of lead-humus complexes decreases. The pH value of the soil solution is one of the most important parameters that determine the amount of sorption of heavy metal ions by the soil. As the pH decreases, the solubility of most heavy metals increases and, consequently, their mobility in the soil solid phase - solution system. J. Esser, N. Bassam (1981 ), while studying the mobility of cadmium in aerobic soil conditions, they found that in the pH range of 4-6, the mobility of cadmium is determined by the ionic strength of the solution, and at pH above 6, sorption by manganese oxides takes on leading importance. Soluble organic compounds, according to the authors, form only weak complexes with cadmium and affect its sorption only at pH 8.
The most mobile and accessible part of heavy metal compounds in the soil is their content in the soil solution. The amount of metal ions entering the soil solution determines the toxicity of a particular element in the soil. The state of equilibrium in the solid phase-solution system determines sorption processes, the nature and direction of which depends on the properties and composition of the soil. The influence of soil properties on the mobility of heavy metals and their transition into water extract is confirmed by data on different quantities water-soluble compounds of Zn, Pb and Cd, transferred from soils with different levels of fertility at the same doses of applied metals (Table 13). Compared to chernozem, more water-soluble metal compounds were contained in soddy-podzolic medium-cultivated soil. The highest content of water-soluble compounds Zn, Pb and Cd was in poorly cultivated soil. Soil cultivation reduced the mobility of heavy metals. In soddy-podzolic poorly cultivated soil, the content of water-soluble forms of Zn. Pb and Cd were 20-35% higher than in average cultivated soil and 1.5-2.0 times higher than in typical chernozem. An increase in soil fertility, accompanied by an increase in the content of humus and phosphates, neutralization of excess acidity and an increase in buffer properties, leads to a decrease in the content of the most aggressive water-soluble form of heavy metals.
The decisive role in the distribution of heavy metals in the soil-solution system is played by the processes of sorption-desorption on the solid phase of the soil, determined by the properties of the soil and independent of the form of the added compound. The resulting compounds of heavy metals with the solid phase of the soil are thermodynamically more stable than the introduced compounds, and they determine the concentration of elements in the soil solution (R.I. Pervunina, 1983).
Soil is a powerful and active absorber of heavy metals; it is able to firmly bind and thereby reduce the flow of toxicants into plants. Mineral and organic components of the soil actively inactivate metal compounds, but the quantitative expressions of their action depend on the type of soil (B A. Bolshakov et al., 1978, V. B. Ilyin, 1987).
The accumulated experimental material indicates that. that the largest amount of heavy metals is extracted from the soil by 1 N acid extract. In this case, the data are close to the total content of elements in the soil. This form of elements can be considered a general reserve quantity capable of being transformed into a mobile, movable form. The content of heavy metal when extracted from the soil with ammonium acetate buffer characterizes the more mobile part. The exchangeable form of the heavy metal is even more mobile. extracted with neutral saline solution. V.S. Gorbatov and N.G. Zyrin (1987) believe that the most accessible form for plants is the exchangeable form of heavy metals, selectively extracted by solutions of salts, the anion of which does not form complexes with heavy metals, and the cation has a high displacing force. These are the properties of Ca(NO3)2 used in our experiment. The most aggressive solvents - acids, most often used 1N HCl and 1N HNO3, extract from the soil not only forms assimilated by plants, but also part of the gross element, which are the closest reserve for transformation into mobile compounds.
The concentration of heavy metals extracted by water extract in the soil solution characterizes the most active part of their compounds. This is the most aggressive and dynamic fraction of heavy metals, characterizing the degree of mobility of elements in the soil. A high content of water-soluble forms of TM can lead not only to contamination of plant products, but also to a sharp reduction in yield, even to its death. With a very high content of the water-soluble form of heavy metal in the soil, it becomes an independent factor determining the size of the crop and the degree of its contamination.
Our country has accumulated information on the content of mobile forms of TM in uncontaminated soils, mainly those known as microelements - Mn, Zn, Cu, Mo. Co (Table 14). To determine the mobile form, individual extractants were most often used (according to Peyve Ya.V. and Rinkis G.Ya.). As can be seen from Table 14, the soils of individual regions differed significantly in the amount of mobile forms of the same metal.

The reason could be, according to V.B. Ilyin (1991), genetic characteristics of soils, primarily the specificity of the granulometric and mineralogical composition, the level of humus content, and the reaction of the environment. For this reason, soils of the same natural region and, moreover, even of the same genetic type within this region can vary greatly.
The difference between the minimum and maximum quantities of mobile form encountered may be within a mathematical order of magnitude. There is absolutely insufficient information on the content of mobile forms of Pb, Cd, Cr, Hg and other most toxic elements in soils. Correctly assessing the mobility of TM in soils is complicated by the use of chemical substances as extractants that vary greatly in their dissolving ability. For example, 1 N HCl extracted mobile forms from the arable horizon in mg/kg: Mn - 414, Zn - 7.8, Ni - 8.3, Cu - 3.5, Pb - 6.8, Co - 5.3 (soil Western Siberia), while 2.5% CH3COOH extracted 76; 0.8; 1.2; 1.3; 0.3; 0.7 (soils of the Tomsk Ob region, data from Ilyin, 1991). These materials indicate that 1 N HCl extracted from the soil, with the exception of zinc, about 30% of the total amount of metals, and 2.5% CH3COOH - less than 10%. Therefore, the extractant 1N HCl, widely used in agrochemical research and soil characterization, has a high mobilizing ability in relation to heavy metal reserves.
The main part of the mobile compounds of heavy metals is confined to humus or root-inhabited soil horizons, in which biochemical processes actively occur and contain a lot of organic substances. Heavy metals. included in organic complexes, have high mobility. V.B. Ilyin (1991) points to the possibility of accumulation of heavy metals in the illuvial and carbonate horizons, into which fine particles saturated with heavy metals and water-soluble forms of elements migrate from the overlying layer. In the illuvial and carbonate horizons, metal-containing compounds precipitate. This is most facilitated by a sharp increase in pH in the soil of these horizons, due to the presence of carbonates.
The ability of heavy metals to accumulate in the lower soil horizons is well illustrated by data on soil profiles in Siberia (Table 15). In the humus horizon, there is an increased content of many elements (Sr, Mn, Zn, Ni, etc.) regardless of their genesis. In many cases, an increase in the content of mobile Sr in the carbonate horizon is clearly visible. The total content of mobile forms in smaller quantities is typical for sandy soils, and in much higher quantities for loamy soils. That is, there is a close connection between the content of mobile forms of elements and the granulometric composition of soils. A similar positive relationship can be seen between the content of mobile forms of heavy metals and the humus content.
The content of mobile forms of heavy metals is subject to strong fluctuations, which is associated with changing biological activity of soils and the influence of plants. Thus, according to research conducted by V.B. Ilyin, the content of mobile molybdenum in soddy-podzolic soil and southern chernozem changed 5 times during the growing season.
In recent years, some research institutions have been studying the effect of long-term use of mineral, organic and lime fertilizers on the content of mobile forms of heavy metals in the soil.
At the Dolgoprudnaya agrochemical experimental station (DAOS, Moscow region), a study was carried out of the accumulation of heavy metals, toxic elements and their mobility in the soil under conditions of long-term use of phosphorus fertilizers on limed sod-podzolic heavy loamy soil (Yu.A. Potatueva et al., 1994 ). The systematic use of ballast and concentrated fertilizers for 60 years, various forms of phosphates for 20 years and phosphate rock from various deposits for 8 years did not have a significant effect on the total content of heavy metals and toxic elements (TE) in the soil, but led to an increase in mobility it contains some TM and TE. The content of mobile and water-soluble forms in the soil increased approximately 2 times with the systematic application of all studied forms of phosphorus fertilizers, however, amounting to only 1/3 of the MPC. The amount of mobile strontium increased 4.5 times in the soil that received simple superphosphate. The addition of raw phosphorites from the Kingisepskoye deposit led to an increase in the content of mobile forms in the soil (AAB pH 4.8): lead by 2 times, nickel by 20% and chromium by 17%, which amounted to 1/4 and 1/10 of the MPC, respectively. An increase in the content of mobile chromium by 17% was noted in the soil that received raw phosphorites from the Chilisay deposit (Table 16).
A comparison of experimental data from long-term field experiments of the DAOS with sanitary and hygienic standards for the content of mobile forms of heavy metals in the soil, and in their absence with the recommendations proposed in the literature, indicates that the content of mobile forms of these elements in the soil was below acceptable levels. These experimental data indicate that even a very long - for 60 years - use of phosphorus fertilizers did not lead to exceeding the maximum permissible concentration level in the soil, either in relation to gross or mobile forms of heavy metals. At the same time, these data indicate that the standardization of heavy metals in soil only by bulk forms is not sufficiently justified and should be supplemented by the content of the mobile form, which reflects both the chemical properties of the metals themselves and the properties of the soil on which plants are grown.
Based on long-term field experience established under the leadership of Academician N.S. Avdonin at the experimental base of Moscow State University "Chashnikovo", a study was conducted of the influence of long-term use of mineral, organic, lime fertilizers and their combinations over 41 years on the content of mobile forms of heavy metals in the soil (V.G. Mineev et al., 1994). The research results presented in Table 17 showed that the creation of optimal conditions for the growth and development of plants significantly reduced the content of mobile forms of lead and cadmium in the soil. The systematic application of nitrogen-potassium fertilizers, acidifying the soil solution and reducing the content of mobile phosphorus, doubled the concentration of mobile compounds of lead and nickel and increased the content of cadmium in the soil by 1.5 times.

The content of bulk and mobile forms of TM in soddy-podzolic light loamy soil of Belarus was studied during long-term use of municipal wastewater sludge: thermophilically digested from sludge fields (TIP) and thermophilically digested with subsequent mechanical dewatering (TMD).
Over 8 years of research, the saturation of OCB crop rotation was 6.25 t/ha (single dose) and 12.5 t/ha (double dose), which is approximately 2-3 times higher than recommended doses.
As can be seen from Table 18, there is a clear pattern of increasing the content of bulk and mobile forms of TM as a result of three-time application of WWS. Moreover, zinc is characterized by the greatest mobility, the amount of which in mobile form increased 3-4 times compared to the control soil (N.P. Reshetsky, 1994). At the same time, the content of mobile compounds of cadmium, copper, lead and chromium did not change significantly.
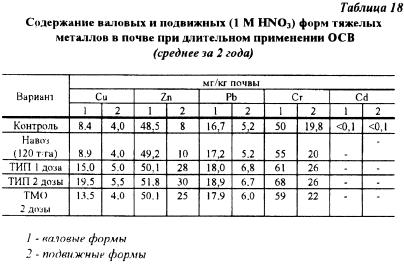
Research by scientists of the Belarusian agricultural sector. Academy showed that when sewage sludge was added (SIP-raw sludge from sludge fields, TIP, TMO), there was a noticeable increase in the content of mobile forms of elements in the soil, but most strongly cadmium, zinc, copper (Table 19). Liming had virtually no effect on the mobility of metals. According to the authors. the use of an extract in 1 N HNO3 to characterize the degree of mobility of metals is not successful, since over 80% of the total content of the element passes into it (A.I. Gorbyleva et al., 1994).

The establishment of certain relationships between changes in the mobility of TM in the soil and the level of acidity was carried out in microfield experiments on leached chernozems of the Central Chernozem Zone of the Russian Federation. At the same time, the determination of cadmium, zinc, lead was carried out in the following extracts: hydrochloric, nitric, sulfuric acids, ammonium acetate buffer at pH 4.8 and pH 3.5, ammonium nitrate, distilled water. A close relationship has been established between the gross content of zinc and its mobile forms extracted by acids R = 0.924-0.948. When using AAB pH 4.8 R=0.784, AAB pH 3.5=0.721. Lead extracted by hydrochloric and nitric acid correlated less closely with the gross content: R=0.64-0.66. Other hoods had much lower correlation coefficients. The correlation between cadmium compounds extracted by acids and gross reserves was very high (R=0.98-0.99). when extracting AAB pH 4.8-R=0.92. The use of other extracts gave results indicating a weak connection between the bulk and mobile forms of heavy metals in the soil (N.P. Bogomazov, P.G. Akulov, 1994).
In a long-term field experiment (All-Russian Research Institute of Flax, Tver Region), with long-term use of fertilizers on sod-podzolic soil, the proportion of mobile metal compounds from the content of their potentially available forms decreased, this is especially noticeable in the 3rd year of the after-effect of lime at a dose of 2 g. (Table . 20). In the 13th year of aftereffect, lime at the same dose reduced only the content of mobile iron and aluminum in the soil. in the 15th year - iron, aluminum and manganese (L.I. Petrova, 1994).

Therefore, to reduce the content of mobile forms of lead and copper in the soil, it is necessary to re-lime the soil.
A study of the mobility of heavy metals in chernozems of the Rostov region showed that in a meter layer of ordinary chernozems, the amount of zinc extracted by ammonium acetate buffer extract with pH 4.8 ranged from 0.26-0.54 mg/kg. manganese 23.1-35.7 mg/kg, copper 0.24-0.42 (G.V. Agafonov, 1994). Comparison of these figures with the gross reserves of microelements in the soil of the same areas showed that the mobility various elements varies significantly. Zinc in carbonate chernozem is 2.5-4.0 times less accessible to plants than copper and 5-8 times less than manganese (Table 21).

Thus, the results of the conducted studies show. that the problem of mobility of heavy metals in soil is complex and multifactorial. The content of mobile forms of heavy metals in the soil depends on many conditions. Main reception, leading to a decrease in the content of this form of heavy metals is an increase in soil fertility (liming, an increase in the content of humus and phosphorus, etc.). At the same time, there is no generally accepted formulation for mobile metals. In this section we offered our understanding of the various fractions of mobile metals in the soil:
1) the total supply of mobile forms (extractable with acids);
2) mobile mobile form (removable with buffer solutions):
3) exchangeable (extracted by neutral salts);
4) water-soluble.
CONTENTS
Introduction
1. Soil cover and its use
2. Soil erosion (water and wind) and methods of combating it
3. Industrial pollution soil
3.1 Acid rain
3.2 Heavy metals
3.3 Lead toxicity
4. Soil hygiene. Waste disposal
4.1 The role of soil in metabolism
4.2 Ecological relationships between soil and water and liquid waste (wastewater)
4.3 Limits of soil load with solid waste (household and street garbage, industrial waste, dry sludge after sewage sedimentation, radioactive substances)
4.4 The role of soil in the spread of various diseases
4.5 Harmful effects of the main types of pollutants (solid and liquid wastes) leading to soil degradation
4.5.1 Neutralization of liquid waste in soil
4.5.2.1 Neutralization of solid waste in soil
4.5.2.2 Garbage collection and removal
4.5.3 Final removal and rendering harmless
4.6 Disposal of radioactive waste
Conclusion
List of sources used
Introduction.
A certain part of the soil, both in Russia and throughout the world, leaves agricultural use every year for various reasons, discussed in detail in the UIR. Thousands or more hectares of land suffer from erosion, acid rain, improper cultivation and toxic waste. To avoid this, you need to become familiar with the most productive and inexpensive reclamation measures (For the definition of reclamation, see the main part of the work) that increase the fertility of the soil cover, and above all with the negative impact on the soil itself, and how to avoid it.
These studies provide insight into the harmful effects on soil and have been conducted through a number of books, articles and scientific journals dealing with soil issues and environmental protection.
The problem of soil pollution and degradation has always been relevant. Now we can add to what has been said that in our time the anthropogenic influence has a strong impact on nature and is only growing, and the soil is one of the main sources of food and clothing for us, not to mention the fact that we walk on it and will always be in close contact with her.
1. Soil cover and its use.
Soil cover is the most important natural formation. Its importance for the life of society is determined by the fact that soil is the main source of food, providing 97-98% of the food resources of the planet's population. At the same time, the soil cover is a place of human activity on which industrial and agricultural production is located.
Highlighting the special role of food in the life of society, V.I. Lenin pointed out: “The real foundations of the economy are the food fund.”
The most important property of the soil cover is its fertility, which is understood as the totality of soil properties that ensure the yield of agricultural crops. Natural soil fertility is regulated by reserve nutrients in the soil and its water, air and thermal regimes. The role of soil cover in the productivity of terrestrial ecological systems is great, since soil nourishes land plants with water and many compounds and is an essential component of the photosynthetic activity of plants. Soil fertility also depends on the amount of solar energy accumulated in it. Living organisms, plants and animals inhabiting the Earth record solar energy in the form of phyto- or zoomass. The productivity of terrestrial ecological systems depends on the thermal and water balance of the earth's surface, which determines the variety of forms of exchange of matter and matter within the geographic envelope of the planet.
Analyzing the importance of land for social production, K. Marx identified two concepts: land-matter and land-capital. The first of these should be understood the earth that arose in the process of its evolutionary development without the will and consciousness of people and is the place of human settlement and the source of his food. From the moment when land, in the process of development of human society, becomes a means of production, it appears in a new quality - capital, without which the labor process is unthinkable, “... because it gives the worker... a place on which he stands... , and its process - the scope of action...”. It is for this reason that the earth is a universal factor in any human activity.
The role and place of land are not the same in various fields material production, primarily in industry and agriculture. In the manufacturing industry, construction, and transport, the earth is the place where labor processes take place regardless of the natural fertility of the soil. Land plays a different role in agriculture. Under the influence of human labor, natural fertility turns from potential into economic. The specificity of the use of land resources in agriculture leads to the fact that they act in two different qualities, as an object of labor and as a means of production. K. Marx noted: “By the mere new investment of capital in plots of land... people increased land-capital without any increase in the matter of the earth, i.e., the space of the earth.”
Land in agriculture acts as a productive force due to its natural fertility, which does not remain constant. With rational use of land, such fertility can be increased by improving its water, air and thermal conditions through reclamation measures and increasing the content of nutrients in the soil. On the contrary, with irrational use of land resources, their fertility decreases, resulting in a decrease in agricultural yields. In some places, cultivation of crops becomes completely impossible, especially on saline and eroded soils.
At a low level of development of the productive forces of society, the expansion of food production occurs due to the involvement of new lands in agriculture, which corresponds to the extensive development of agriculture. This is facilitated by two conditions: the availability of free land and the possibility of farming at an affordable average level of capital costs per unit area. This use of land resources and farming is typical of many developing countries in the modern world.
During the era of scientific and technological revolution, there was a sharp distinction between the farming system in industrialized and developing countries. The former are characterized by the intensification of agriculture using the achievements of scientific and technological revolution, in which agriculture develops not due to an increase in the area of cultivated land, but due to an increase in the amount of capital invested in the land. The well-known limitation of land resources for most industrialized capitalist countries, the increasing demand for agricultural products throughout the world due to high rates of population growth, and a higher culture of agriculture contributed to the transfer of agriculture in these countries back to the 50s on the path of intensive development. The acceleration of the process of intensification of agriculture in industrialized capitalist countries is associated not only with the achievements of scientific and technological revolution, but mainly with the profitability of investing capital in agriculture, which concentrated agricultural production in the hands of large landowners and ruined small farmers.
Agriculture developed in other ways in developing countries. Among the acute natural resource problems of these countries, the following can be identified: low agricultural standards, which caused degradation of soils (increased erosion, salinization, decreased fertility) and natural vegetation (for example, tropical forests), depletion of water resources, desertification of lands, especially clearly manifested in African countries. continent. All these factors related to the socio-economic problems of developing countries have led to chronic food shortages in these countries. Thus, at the beginning of the 80s, in terms of provision per person with grain (222 kg) and meat (14 kg), developing countries were inferior to industrialized capitalist countries, respectively, several times. Solving the food problem in developing countries is unthinkable without major socio-economic transformations.
In our country, the basis of land relations is the national (national) ownership of land, which arose as a result of the nationalization of all land. Agrarian relations are built on the basis of plans according to which agriculture should develop in the future, with financial and credit assistance from the state and the supply of the required number of machines and fertilizers. Paying agricultural workers according to the quantity and quality of work stimulates a constant increase in their living standards.
The use of the land fund as a whole is carried out on the basis of long-term state plans. An example of such plans was the development of virgin and fallow lands in the east of the country (mid-50s), thanks to which it became possible to introduce more than 41 million hectares of new areas into arable land in a short period of time. Another example is a set of measures related to the implementation of the Food Program, which provides for accelerating the development of agricultural production based on improving farming standards, extensive land reclamation activities, as well as the implementation of a broad program of socio-economic reconstruction of agricultural areas.
The world's land resources as a whole make it possible to provide food for more people than is currently available and will be the case in the near future. At the same time, due to population growth, especially in developing countries, the amount of arable land per capita is decreasing.
PAGE_BREAK-- heavy metals, which characterizes a wide group of pollutants, has recently become widespread. In various scientific and applied works, authors interpret the meaning of this concept differently. In this regard, the amount of elements classified as heavy metals varies widely. Numerous characteristics are used as membership criteria: atomic mass, density, toxicity, prevalence in the natural environment, degree of involvement in natural and man-made cycles. In some cases, the definition of heavy metals includes elements classified as brittle (for example, bismuth) or metalloids (for example, arsenic).
In works devoted to the problems of environmental pollution natural environment and environmental monitoring, today to heavy metals include more than 40 metals of the periodic table D.I. Mendeleev with an atomic mass of over 50 atomic units: V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Mo, Cd, Sn, Hg, Pb, Bi etc. At the same time, the following conditions play an important role in the categorization of heavy metals: their high toxicity to living organisms in relatively low concentrations, as well as the ability to bioaccumulate and biomagnify. Almost all metals that fall under this definition (with the exception of lead, mercury, cadmium and bismuth, the biological role of which is currently unclear) are actively involved in biological processes and are part of many enzymes. According to the classification of N. Reimers, metals with a density of more than 8 g/cm3 should be considered heavy. Thus, heavy metals include Pb, Cu, Zn, Ni, Cd, Co, Sb, Sn, Bi, Hg.
Formally defined heavy metals corresponds to a large number of elements. However, according to researchers engaged in practical activities related to organizing observations of the state and pollution of the environment, compounds of these elements are far from equivalent as pollutants. Therefore, in many works, the scope of the group of heavy metals is narrowed, in accordance with priority criteria determined by the direction and specifics of the work. Thus, in the now classic works of Yu.A. Israel in the list of chemical substances to be determined in natural environments at background stations in biosphere reserves, in section heavy metals named Pb, Hg, Cd, As. On the other hand, according to the decision of the Task Force on Heavy Metal Emissions, working under the auspices of the United Nations Economic Commission for Europe and collecting and analyzing information on pollutant emissions in European countries, only Zn, As, Se and Sb were attributed to heavy metals. According to N. Reimers’ definition, noble and rare metals stand apart from heavy metals, respectively, they remain only Pb, Cu, Zn, Ni, Cd, Co, Sb, Sn, Bi, Hg. In applied work, heavy metals are most often added Pt, Ag, W, Fe, Au, Mn.
Metal ions are essential components of natural bodies of water. Depending on environmental conditions (pH, redox potential, presence of ligands), they exist in different oxidation states and are part of a variety of inorganic and organometallic compounds, which can be truly dissolved, colloidal dispersed, or part of mineral and organic suspensions.
Truly dissolved forms of metals, in turn, are very diverse, which is associated with the processes of hydrolysis, hydrolytic polymerization (formation of polynuclear hydroxo complexes) and complexation with various ligands. Accordingly, both the catalytic properties of metals and their availability for aquatic microorganisms depend on the forms of their existence in the aquatic ecosystem.
Many metals form fairly strong complexes with organic matter; These complexes are one of the most important forms of migration of elements in natural waters. Most organic complexes are formed via the chelate cycle and are stable. Complexes formed by soil acids with salts of iron, aluminum, titanium, uranium, vanadium, copper, molybdenum and other heavy metals are relatively well soluble in neutral, slightly acidic and slightly alkaline environments. Therefore, organometallic complexes are capable of migrating in natural waters over very long distances. This is especially important for low-mineralized and primarily surface waters, in which the formation of other complexes is impossible.
To understand the factors that regulate the concentration of metal in natural waters, their chemical reactivity, bioavailability and toxicity, it is necessary to know not only the total content, but also the proportion of free and related forms metal
The transition of metals in an aqueous environment into a metal complex form has three consequences:
1. An increase in the total concentration of metal ions may occur due to its transition into solution from bottom sediments;
2. The membrane permeability of complex ions can differ significantly from the permeability of hydrated ions;
3. The toxicity of the metal may change greatly as a result of complexation.
So, chelate forms Cu, Cd, Hg less toxic than free ions. To understand the factors that regulate the concentration of the metal in natural waters, their chemical reactivity, bioavailability and toxicity, it is necessary to know not only the total content, but also the proportion of bound and free forms.
Sources of water pollution with heavy metals are wastewater galvanizing shops, mining enterprises, ferrous and non-ferrous metallurgy, machine-building plants. Heavy metals are found in fertilizers and pesticides and can enter water bodies through agricultural runoff.
Increased concentrations of heavy metals in natural waters are often associated with other types of pollution, such as acidification. Acid precipitation contributes to a decrease in pH and the transition of metals from a state sorbed on mineral and organic substances to a free state.
First of all, those metals of interest are those that most pollute the atmosphere due to their use in significant quantities in industrial activities and, as a result of accumulation in the external environment, pose a serious danger in terms of their biological activity and toxic properties. These include lead, mercury, cadmium, zinc, bismuth, cobalt, nickel, copper, tin, antimony, vanadium, manganese, chromium, molybdenum and arsenic.
Biogeochemical properties of heavy metals
V - high, U - moderate, N - low
Vanadium.
Vanadium is predominantly in a dispersed state and is found in iron ores, oil, asphalt, bitumen, oil shale, coal, etc. One of the main sources of pollution of natural waters with vanadium is oil and its refined products.
In natural waters it occurs in very low concentrations: in river water 0.2 - 4.5 μg/dm3, in sea water - on average 2 μg/dm3
In water it forms stable anionic complexes (V4O12)4- and (V10O26)6-. In the migration of vanadium, the role of dissolved complex compounds with organic substances, especially with humic acids, is significant.
Elevated concentrations of vanadium are harmful to human health. The MPC of vanadium is 0.1 mg/dm3 (the limiting hazard indicator is sanitary-toxicological), the MPCv is 0.001 mg/dm3.
Natural sources of bismuth entering natural waters are the processes of leaching of bismuth-containing minerals. The source of entry into natural waters can also be wastewater from pharmaceutical and perfume production, and some glass industry enterprises.
It is found in submicrogram concentrations in unpolluted surface waters. The highest concentration was found in groundwater and is 20 μg/dm3, in sea waters- 0.02 µg/dm3. MPC is 0.1 mg/dm3
The main sources of iron compounds in surface waters are the processes of chemical weathering of rocks, accompanied by their mechanical destruction and dissolution. In the process of interaction with mineral and organic substances contained in natural waters, a complex complex of iron compounds is formed, which are in the water in a dissolved, colloidal and suspended state. Significant amounts of iron come from underground runoff and wastewater from metallurgical, metalworking, textile, paint and varnish industries and agricultural runoff.
Phase equilibria depend on the chemical composition of water, pH, Eh and, to some extent, temperature. In routine analysis weighted form emit particles larger than 0.45 microns. It consists predominantly of iron-containing minerals, iron oxide hydrate and iron compounds sorbed in suspensions. The truly dissolved and colloidal forms are usually considered together. Dissolved iron is represented by compounds in ionic form, in the form of a hydroxo complex and complexes with dissolved inorganic and organic substances of natural waters. It is mainly Fe(II) that migrates in ionic form, and Fe(III) in the absence of complexing substances cannot be in a dissolved state in significant quantities.
Iron is found mainly in waters with low Eh values.
As a result of chemical and biochemical (with the participation of iron bacteria) oxidation, Fe(II) transforms into Fe(III), which, when hydrolyzed, precipitates in the form of Fe(OH)3. Both Fe(II) and Fe(III) are characterized by a tendency to form hydroxo complexes of the type +, 4+, +, 3+, - and others, coexisting in solution in different concentrations depending on pH and generally determining the state of the iron-hydroxyl system. The main form of Fe(III) in surface waters is its complex compounds with dissolved inorganic and organic compounds, mainly humic substances. At pH = 8.0, the main form is Fe(OH)3. The colloidal form of iron is the least studied; it consists of iron oxide hydrate Fe(OH)3 and complexes with organic substances.
The iron content in surface waters of land is tenths of a milligram; near swamps it is a few milligrams. An increased iron content is observed in swamp waters, in which it is found in the form of complexes with salts of humic acids - humates. The highest concentrations of iron (up to several tens and hundreds of milligrams per 1 dm3) are observed in groundwater with low pH values.
Being a biologically active element, iron to a certain extent affects the intensity of phytoplankton development and the qualitative composition of microflora in a reservoir.
Iron concentrations are subject to marked seasonal fluctuations. Typically, in reservoirs with high biological productivity during the period of summer and winter stagnation, there is a noticeable increase in the concentration of iron in the bottom layers of water. Autumn-spring mixing of water masses (homothermy) is accompanied by the oxidation of Fe(II) to Fe(III) and the precipitation of the latter in the form of Fe(OH)3.
It enters natural waters through the leaching of soils, polymetallic and copper ores, as a result of the decomposition of aquatic organisms capable of accumulating it. Cadmium compounds are carried into surface waters with wastewater from lead-zinc plants, ore processing plants, a number of chemical enterprises (sulfuric acid production), galvanic production, and also with mine waters. A decrease in the concentration of dissolved cadmium compounds occurs due to the processes of sorption, precipitation of cadmium hydroxide and carbonate and their consumption by aquatic organisms.
Dissolved forms of cadmium in natural waters are mainly mineral and organomineral complexes. The main suspended form of cadmium is its sorbed compounds. A significant portion of cadmium can migrate within the cells of aquatic organisms.
In unpolluted and slightly polluted river waters, cadmium is contained in submicrogram concentrations; in polluted and waste waters, the concentration of cadmium can reach tens of micrograms per 1 dm3.
Cadmium compounds play an important role in the life processes of animals and humans. In elevated concentrations it is toxic, especially in combination with other toxic substances.
The maximum permissible concentration is 0.001 mg/dm3, the maximum permissible concentration is 0.0005 mg/dm3 (the limiting sign of harm is toxicological).
Cobalt compounds enter natural waters as a result of leaching processes from copper pyrite and other ores, from soils during the decomposition of organisms and plants, as well as with wastewater from metallurgical, metalworking and chemical plants. Some amounts of cobalt come from soils as a result of decomposition of plant and animal organisms.
Cobalt compounds in natural waters are in a dissolved and suspended state, the quantitative relationship between which is determined by the chemical composition of the water, temperature and pH values. Dissolved forms are represented mainly by complex compounds, incl. with organic substances of natural waters. Compounds of divalent cobalt are most typical for surface waters. In the presence of oxidizing agents, trivalent cobalt can exist in noticeable concentrations.
Cobalt is one of the biologically active elements and is always found in the body of animals and plants. Insufficient cobalt content in soils is associated with insufficient cobalt content in plants, which contributes to the development of anemia in animals (taiga-forest non-chernozem zone). As part of vitamin B12, cobalt very actively influences the supply of nitrogenous substances, increases the content of chlorophyll and ascorbic acid, activates biosynthesis and increases the content of protein nitrogen in plants. However, increased concentrations of cobalt compounds are toxic.
In unpolluted and slightly polluted river waters, its content ranges from tenths to thousandths of a milligram per 1 dm3, the average content in sea water is 0.5 μg/dm3. The maximum permissible concentration is 0.1 mg/dm3, the maximum permissible concentration is 0.01 mg/dm3.
Manganese
Manganese enters surface waters as a result of leaching of ferromanganese ores and other minerals containing manganese (pyrolusite, psilomelane, braunite, manganite, black ochre). Significant amounts of manganese come from the decomposition of aquatic animals and plant organisms, especially blue-greens, diatoms and higher aquatic plants. Manganese compounds are carried into reservoirs with wastewater from manganese enrichment factories, metallurgical plants, chemical industry enterprises and mine waters.
A decrease in the concentration of manganese ions in natural waters occurs as a result of the oxidation of Mn(II) to MnO2 and other high-valent oxides that precipitate. The main parameters that determine the oxidation reaction are the concentration of dissolved oxygen, pH value and temperature. The concentration of dissolved manganese compounds decreases due to their utilization by algae.
The main form of migration of manganese compounds in surface waters is suspensions, the composition of which is determined in turn by the composition of the rocks drained by the waters, as well as colloidal hydroxides of heavy metals and sorbed manganese compounds. Organic substances and the processes of complex formation of manganese with inorganic and organic ligands are of significant importance in the migration of manganese in dissolved and colloidal forms. Mn(II) forms soluble complexes with bicarbonates and sulfates. Complexes of manganese with chlorine ions are rare. Complex compounds of Mn(II) with organic substances are usually less stable than with other transition metals. These include compounds with amines, organic acids, amino acids and humic substances. Mn(III) in high concentrations can be in a dissolved state only in the presence of strong complexing agents; Mn(YII) is not found in natural waters.
In river waters, the manganese content usually ranges from 1 to 160 μg/dm3, the average content in sea waters is 2 μg/dm3, in underground waters - n.102 - n.103 μg/dm3.
Manganese concentrations in surface waters are subject to seasonal fluctuations.
The factors that determine changes in manganese concentrations are the ratio between surface and underground runoff, the intensity of its consumption during photosynthesis, the decomposition of phytoplankton, microorganisms and higher aquatic vegetation, as well as the processes of its deposition to the bottom of water bodies.
The role of manganese in the life of higher plants and algae in water bodies is very large. Manganese promotes the utilization of CO2 by plants, which increases the intensity of photosynthesis and participates in the processes of nitrate reduction and nitrogen assimilation by plants. Manganese promotes the transition of active Fe(II) to Fe(III), which protects the cell from poisoning, accelerates the growth of organisms, etc. The important ecological and physiological role of manganese necessitates the study and distribution of manganese in natural waters.
For reservoirs for sanitary use, the maximum permissible concentration (MPC) (for manganese ion) is set to 0.1 mg/dm3.
Below are maps of the distribution of average concentrations of metals: manganese, copper, nickel and lead, constructed according to observational data for 1989 - 1993. in 123 cities. The use of more recent data is assumed to be inappropriate, since due to the reduction in production, the concentrations of suspended substances and, accordingly, metals have significantly decreased.
Impact on health. Many metals are part of dust and have a significant impact on health.
Manganese enters the atmosphere from emissions from ferrous metallurgy (60% of all manganese emissions), mechanical engineering and metalworking (23%), non-ferrous metallurgy (9%), and numerous small sources, for example, from welding.
High concentrations of manganese lead to neurotoxic effects, progressive damage to the central nervous system, pneumonia.
The highest concentrations of manganese (0.57 - 0.66 μg/m3) are observed in large centers of metallurgy: Lipetsk and Cherepovets, as well as Magadan. Most cities with high concentrations of Mn (0.23 - 0.69 μg/m3) are concentrated on the Kola Peninsula: Zapolyarny, Kandalaksha, Monchegorsk, Olenegorsk (see map).
For 1991 - 1994 manganese emissions from industrial sources decreased by 62%, average concentrations by 48%.
Copper is one of the most important trace elements. The physiological activity of copper is associated mainly with its inclusion in the active centers of redox enzymes. Insufficient copper content in soils negatively affects the synthesis of proteins, fats and vitamins and contributes to the infertility of plant organisms. Copper is involved in the process of photosynthesis and affects the absorption of nitrogen by plants. At the same time, excessive concentrations of copper have an adverse effect on plant and animal organisms.
Cu(II) compounds are most common in natural waters. Of the Cu(I) compounds, the most common are Cu2O, Cu2S, and CuCl, which are sparingly soluble in water. In the presence of ligands in an aqueous medium, along with the equilibrium of hydroxide dissociation, it is necessary to take into account the formation of various complex forms that are in equilibrium with metal aqua ions.
The main source of copper entering natural waters is wastewater from chemical and metallurgical industries, mine water, and aldehyde reagents used to destroy algae. Copper can result from corrosion of copper piping and other structures used in water supply systems. In groundwater, the copper content is determined by the interaction of water with rocks containing it (chalcopyrite, chalcocite, covellite, bornite, malachite, azurite, chrysacolla, brotantine).
The maximum permissible concentration of copper in the water of reservoirs for sanitary water use is 0.1 mg/dm3 (the limiting sign of hazard is general sanitary), in the water of fishery reservoirs - 0.001 mg/dm3.
City
Norilsk
Monchegorsk
Krasnouralsk
Kolchugino
Zapolyarny
Emissions M (thousand tons/year) of copper oxide and average annual concentrations q (μg/m3) of copper.
Copper enters the air with emissions from metallurgical production. In solid emissions it is contained mainly in the form of compounds, mainly copper oxide.
Non-ferrous metallurgy enterprises account for 98.7% of all anthropogenic emissions of this metal, of which 71% are carried out by enterprises of the Norilsk Nickel concern located in Zapolyarny and Nikel, Monchegorsk and Norilsk, and approximately 25% of copper emissions are carried out in Revda and Krasnouralsk , Kolchugino and others.
High concentrations of copper lead to intoxication, anemia and hepatitis.
As can be seen from the map, the highest concentrations of copper were noted in the cities of Lipetsk and Rudnaya Pristan. Copper concentrations have also increased in the cities of the Kola Peninsula, in Zapolyarny, Monchegorsk, Nikel, Olenegorsk, as well as in Norilsk.
Copper emissions from industrial sources decreased by 34%, average concentrations by 42%.
Molybdenum
Molybdenum compounds enter surface waters as a result of leaching from exogenous molybdenum-containing minerals. Molybdenum also enters water bodies with wastewater from processing plants and non-ferrous metallurgy enterprises. A decrease in the concentrations of molybdenum compounds occurs as a result of precipitation of sparingly soluble compounds, adsorption processes by mineral suspensions and consumption by plant aquatic organisms.
Molybdenum in surface waters is mainly in the form MoO42-. It is very likely that it exists in the form of organomineral complexes. The possibility of some accumulation in the colloidal state follows from the fact that the oxidation products of molybdenite are loose, finely dispersed substances.
In river waters, molybdenum was found in concentrations from 2.1 to 10.6 μg/dm3. Sea water contains an average of 10 µg/dm3 of molybdenum.
In small quantities, molybdenum is necessary for the normal development of plant and animal organisms. Molybdenum is part of the enzyme xanthine oxidase. With molybdenum deficiency, the enzyme is formed in insufficient quantities, which causes negative reactions in the body. In elevated concentrations, molybdenum is harmful. With an excess of molybdenum, metabolism is disrupted.
The maximum permissible concentration of molybdenum in water bodies for sanitary use is 0.25 mg/dm3.
Arsenic enters natural waters from mineral springs, areas of arsenic mineralization (arsenic pyrite, realgar, orpiment), as well as from zones of oxidation of polymetallic, copper-cobalt and tungsten rocks. Some arsenic comes from soils and also from decomposition of plant and animal organisms. The consumption of arsenic by aquatic organisms is one of the reasons for the decrease in its concentration in water, which is most clearly manifested during the period of intensive plankton development.
Significant amounts of arsenic enter water bodies from wastewater from processing plants, dye production waste, tanneries and pesticide plants, as well as from agricultural lands where pesticides are used.
In natural waters, arsenic compounds are in a dissolved and suspended state, the relationship between which is determined by the chemical composition of the water and pH values. In dissolved form, arsenic occurs in tri- and pentavalent forms, mainly as anions.
In unpolluted river waters, arsenic is usually found in microgram concentrations. In mineral waters its concentration can reach several milligrams per 1 dm3, in sea waters it contains an average of 3 µg/dm3, in underground waters it is found in concentrations of n.105 µg/dm3. Arsenic compounds in high concentrations are toxic to the body of animals and humans: they inhibit oxidative processes and inhibit the oxygen supply to organs and tissues.
The maximum permissible concentration for arsenic is 0.05 mg/dm3 (the limiting hazard indicator is sanitary-toxicological) and the maximum permissible concentration for arsenic is 0.05 mg/dm3.
The presence of nickel in natural waters is due to the composition of the rocks through which the water passes: it is found in places where sulfide copper-nickel ores and iron-nickel ores are deposited. It enters water from soils and from plant and animal organisms during their decay. Increased nickel content compared to other types of algae was found in blue-green algae. Nickel compounds also enter water bodies with wastewater from nickel plating shops, synthetic rubber plants, and nickel concentration factories. Huge nickel emissions accompany the burning of fossil fuels.
Its concentration may decrease as a result of the precipitation of compounds such as cyanides, sulfides, carbonates or hydroxides (with increasing pH values), due to its consumption by aquatic organisms and adsorption processes.
In surface waters, nickel compounds are in dissolved, suspended and colloidal states, the quantitative ratio between which depends on the composition of the water, temperature and pH values. Sorbents for nickel compounds can be iron hydroxide, organic substances, highly dispersed calcium carbonate, and clays. Dissolved forms are primarily complex ions, most commonly with amino acids, humic and fulvic acids, and also as a strong cyanide complex. The most common nickel compounds in natural waters are those in which it is found in the +2 oxidation state. Ni3+ compounds are usually formed in an alkaline environment.
Nickel compounds play an important role in hematopoietic processes, being catalysts. Its increased content has a specific effect on the cardiovascular system. Nickel is one of the carcinogenic elements. It can cause respiratory diseases. It is believed that free nickel ions (Ni2+) are approximately 2 times more toxic than its complex compounds.
In unpolluted and slightly polluted river waters, the concentration of nickel usually ranges from 0.8 to 10 μg/dm3; in polluted ones it amounts to several tens of micrograms per 1 dm3. The average concentration of nickel in sea water is 2 μg/dm3, in groundwater - n.103 μg/dm3. In groundwater washing nickel-containing rocks, the concentration of nickel sometimes increases to 20 mg/dm3.
Nickel enters the atmosphere from non-ferrous metallurgy enterprises, which account for 97% of all nickel emissions, of which 89% come from enterprises of the Norilsk Nickel concern located in Zapolyarny and Nikel, Monchegorsk and Norilsk.
Increased nickel content in the environment leads to the emergence of endemic diseases, bronchial cancer. Nickel compounds belong to group 1 carcinogens.
The map shows several points with high average nickel concentrations in the locations of the Norilsk Nickel concern: Apatity, Kandalaksha, Monchegorsk, Olenegorsk.
Nickel emissions from industrial enterprises decreased by 28%, average concentrations by 35%.
Emissions M (thousand tons/year) and average annual concentrations q (µg/m3) of nickel.
It enters natural waters as a result of leaching processes of tin-containing minerals (cassiterite, stannin), as well as with wastewater from various industries (dying of fabrics, synthesis of organic paints, production of alloys with the addition of tin, etc.).
The toxic effect of tin is small.
In unpolluted surface waters, tin is found in submicrogram concentrations. In groundwater its concentration reaches a few micrograms per 1 dm3. The maximum permissible concentration is 2 mg/dm3.
Mercury compounds can enter surface waters as a result of leaching of rocks in the area of mercury deposits (cinnabar, metacinnabarite, livingstonite), during the decomposition of aquatic organisms that accumulate mercury. Significant quantities enter water bodies with wastewater from enterprises producing dyes, pesticides, pharmaceuticals, and some explosives. Coal-fired thermal power plants emit significant amounts of mercury compounds into the atmosphere, which end up in water bodies as a result of wet and dry deposition.
A decrease in the concentration of dissolved mercury compounds occurs as a result of their extraction by many marine and freshwater organisms, which have the ability to accumulate it in concentrations many times higher than its content in water, as well as adsorption processes by suspended substances and bottom sediments.
In surface waters, mercury compounds are in a dissolved and suspended state. The ratio between them depends on the chemical composition of the water and pH values. Suspended mercury is sorbed mercury compounds. Dissolved forms are undissociated molecules, complex organic and mineral compounds. Mercury can be present in the water of water bodies in the form of methylmercury compounds.
Mercury compounds are highly toxic, they affect the human nervous system, cause changes in the mucous membrane, impaired motor function and secretion of the gastrointestinal tract, changes in the blood, etc. Bacterial methylation processes are aimed at the formation of methylmercury compounds, which are many times more toxic than mineral salts mercury Methylmercury compounds accumulate in fish and can enter the human body.
The maximum permissible concentration of mercury is 0.0005 mg/dm3 (the limiting sign of hazard is sanitary-toxicological), the maximum permissible concentration is 0.0001 mg/dm3.
Natural sources of lead entering surface waters are the dissolution processes of endogenous (galena) and exogenous (anglesite, cerussite, etc.) minerals. A significant increase in the content of lead in the environment (including in surface waters) is associated with the combustion of coal, the use of tetraethyl lead as an anti-knock agent in motor fuel, and the discharge into water bodies with wastewater from ore processing factories, some metallurgical plants, chemical plants, mines, etc. Significant factors in reducing the concentration of lead in water are its adsorption by suspended substances and precipitation with them into bottom sediments. Lead, among other metals, is extracted and accumulated by aquatic organisms.
Lead is found in natural waters in a dissolved and suspended (sorbed) state. In dissolved form it is found in the form of mineral and organomineral complexes, as well as simple ions, in insoluble form - mainly in the form of sulfides, sulfates and carbonates.
In river waters, the concentration of lead ranges from tenths to units of micrograms per 1 dm3. Even in the water of water bodies adjacent to areas of polymetallic ores, its concentration rarely reaches tens of milligrams per 1 dm3. Only in chloride thermal waters does the concentration of lead sometimes reach several milligrams per 1 dm3.
The limiting indicator of the harmfulness of lead is sanitary-toxicological. The maximum permissible concentration for lead is 0.03 mg/dm3, the maximum permissible concentration for lead is 0.1 mg/dm3.
Lead is contained in emissions from metallurgy, metalworking, electrical engineering, petrochemical and motor transport enterprises.
The impact of lead on health occurs through inhalation of lead-containing air and ingestion of lead through food, water, and dust particles. Lead accumulates in the body, in bones and surface tissues. Lead affects the kidneys, liver, nervous system and blood-forming organs. The elderly and children are especially sensitive to even low doses of lead.
Emissions M (thousand tons/year) and average annual concentrations q (µg/m3) of lead.
Over seven years, lead emissions from industrial sources fell by 60% due to production cuts and many plant closures. The sharp decrease in industrial emissions is not accompanied by a decrease in vehicle emissions. Average lead concentrations decreased by only 41%. The differences in lead emission reductions and concentrations may be explained by under-reporting of vehicle emissions in previous years; Currently, the number of cars and the intensity of their traffic have increased.
Tetraethyl lead
It enters natural waters due to its use as an antiknock agent in motor fuel of water vehicles, as well as with surface runoff from urban areas.
This substance is characterized by high toxicity and has cumulative properties.
The sources of silver entering surface waters are groundwater and wastewater from mines, processing plants, and photographic enterprises. Increased silver content is associated with the use of bactericidal and algicidal preparations.
In wastewater, silver can be present in dissolved and suspended form, mostly in the form of halide salts.
In unpolluted surface waters, silver is found in submicrogram concentrations. In groundwater, the concentration of silver ranges from a few to tens of micrograms per 1 dm3, in sea water - on average 0.3 μg/dm3.
Silver ions are capable of destroying bacteria and even in small concentrations they sterilize water (the lower limit of the bactericidal effect of silver ions is 2.10-11 mol/dm3). The role of silver in the body of animals and humans has not been sufficiently studied.
The MPC of silver is 0.05 mg/dm3.
Antimony enters surface waters due to the leaching of antimony minerals (stibnite, senarmontite, valentinite, servantite, stibiocanite) and with wastewater from rubber, glass, dyeing, and match factories.
In natural waters, antimony compounds are in a dissolved and suspended state. Under the redox conditions characteristic of surface waters, the existence of both trivalent and pentavalent antimony is possible.
In unpolluted surface waters, antimony is found in submicrogram concentrations, in sea water its concentration reaches 0.5 μg/dm3, in groundwater - 10 μg/dm3. The MPC of antimony is 0.05 mg/dm3 (the limiting hazard indicator is sanitary-toxicological), the MPCv is 0.01 mg/dm3.
Tri- and hexavalent chromium compounds enter surface waters as a result of leaching from rocks (chromite, crocoite, uvarovite, etc.). Some amounts come from the decomposition of organisms and plants from soils. Significant quantities may enter water bodies with wastewater from electroplating shops, dyeing shops of textile factories, tanneries and chemical industry enterprises. A decrease in the concentration of chromium ions can be observed as a result of their consumption by aquatic organisms and adsorption processes.
In surface waters, chromium compounds are in dissolved and suspended states, the ratio between which depends on the composition of the water, temperature, and pH of the solution. Suspended chromium compounds are mainly sorbed chromium compounds. Sorbents can be clays, iron hydroxide, highly dispersed settling calcium carbonate, remains of plant and animal organisms. In dissolved form, chromium can be found in the form of chromates and dichromates. Under aerobic conditions, Cr(VI) transforms into Cr(III), the salts of which hydrolyze in neutral and alkaline media to release hydroxide.
In unpolluted and slightly polluted river waters, the chromium content ranges from a few tenths of a microgram per liter to several micrograms per liter; in polluted water bodies it reaches several tens and hundreds of micrograms per liter. The average concentration in sea waters is 0.05 µg/dm3, in groundwater - usually within the range of n.10 - n.102 µg/dm3.
Compounds of Cr(VI) and Cr(III) in increased quantities have carcinogenic properties. Cr(VI) compounds are more dangerous.
It enters natural waters as a result of the processes of destruction and dissolution of rocks and minerals occurring in nature (sphalerite, zincite, goslarite, smithsonite, calamine), as well as with wastewater from ore processing factories and electroplating shops, production of parchment paper, mineral paints, viscose fiber and etc.
In water it exists mainly in ionic form or in the form of its mineral and organic complexes. Sometimes found in insoluble forms: as hydroxide, carbonate, sulfide, etc.
In river waters, the concentration of zinc usually ranges from 3 to 120 μg/dm3, in sea waters - from 1.5 to 10 μg/dm3. The content in ore waters and especially in mine waters with low pH values can be significant.
Zinc is one of the active microelements that influence the growth and normal development of organisms. At the same time, many zinc compounds are toxic, primarily its sulfate and chloride.
The MPC for Zn2+ is 1 mg/dm3 (the limiting indicator of harm is organoleptic), the MPC for Zn2+ is 0.01 mg/dm3 (the limiting indicator of harm is toxicological).
Heavy metals already occupy the second place in terms of danger, inferior to pesticides and significantly ahead of such well-known pollutants as carbon dioxide and sulfur, and in the forecast they should become the most dangerous, more dangerous than nuclear power plant waste and solid waste. Pollution with heavy metals is associated with their widespread use in industrial production, coupled with weak purification systems, as a result of which heavy metals enter the environment, including the soil, polluting and poisoning it.
Heavy metals are priority pollutants, monitoring of which is mandatory in all environments. In various scientific and applied works, authors interpret the meaning of the concept of “heavy metals” differently. In some cases, the definition of heavy metals includes elements classified as brittle (for example, bismuth) or metalloids (for example, arsenic).
Soil is the main medium into which heavy metals enter, including from the atmosphere and the aquatic environment. It also serves as a source of secondary pollution of surface air and waters that flow from it into the World Ocean. From the soil, heavy metals are absorbed by plants, which then become food for more highly organized animals.
continuation
--PAGE_BREAK-- 3.3. Lead toxicity
Currently, lead ranks first among the causes of industrial poisoning. This is due to its widespread use in various industries. Workers who mine lead ore, lead smelters, battery manufacturing, soldering, printing, crystal glass or ceramics, leaded gasoline, lead paint, etc. are exposed to lead. atmospheric air, soil and water in the vicinity of such industries, as well as near major highways, creates a threat of lead damage to the population living in these areas, and, above all, children, who are more sensitive to the effects of heavy metals.
It should be noted with regret that in Russia there is no state policy on legal, regulatory and economic regulation of the impact of lead on the environment and public health, on reducing emissions (discharges, waste) of lead and its compounds into the environment, and on completely stopping the production of lead-containing gasoline.
Due to extremely unsatisfactory educational work to explain to the population the degree of danger of the effects of heavy metals on the human body, in Russia the number of contingents with professional contact with lead is not decreasing, but is gradually increasing. Cases of chronic lead intoxication have been recorded in 14 industries in Russia. The leading industries are the electrical industry (battery production), instrument making, printing and non-ferrous metallurgy, in them, intoxication is caused by exceeding the maximum permissible concentration (MPC) of lead in the air of the working area by 20 or more times.
A significant source of lead is automobile exhaust fumes, as half of Russia still uses leaded gasoline. However, metallurgical plants, in particular copper smelters, remain the main source of environmental pollution. And there are leaders here. In the territory Sverdlovsk region There are 3 of the largest sources of lead emissions in the country: in the cities of Krasnouralsk, Kirovograd and Revda.
The chimneys of the Krasnouralsk copper smelter, built during the years of Stalinist industrialization and using equipment from 1932, annually spew 150-170 tons of lead into the city of 34,000, covering everything with lead dust.
The concentration of lead in the soil of Krasnouralsk varies from 42.9 to 790.8 mg/kg with a maximum permissible concentration of MPC = 130 μ/kg. Water samples in the water supply of a neighboring village. Oktyabrsky, fed by an underground water source, exceeded the maximum permissible concentration by up to two times.
Lead pollution of the environment affects human health. Exposure to lead disrupts the female and male reproductive systems. For women of pregnant and childbearing age, elevated levels of lead in the blood pose a particular danger, since under the influence of lead menstrual function is disrupted, premature births, miscarriages and fetal death are more common due to the penetration of lead through the placental barrier. Newborn babies have a high mortality rate.
Lead poisoning is extremely dangerous for young children - it affects the development of the brain and nervous system. Testing of 165 Krasnouralsk children aged 4 years and older revealed a significant delay in mental development in 75.7%, and mental retardation, including mental retardation, was found in 6.8% of the children examined.
Children preschool age are most susceptible to the harmful effects of lead because their nervous systems are still developing. Even at low doses, lead poisoning causes a decrease in intellectual development, attention and ability to concentrate, a lag in reading, and leads to the development of aggressiveness, hyperactivity and other problems in the child’s behavior. These developmental abnormalities can be long-lasting and irreversible. Low birth weight, stunting and hearing loss also result from lead poisoning. High doses of intoxication lead to mental retardation, coma, convulsions and death.
A white paper published by Russian experts reports that lead pollution covers the entire country and is one of numerous environmental disasters in the former Soviet Union that have come to light in recent years. Most of the territory of Russia experiences a load from lead deposition that exceeds the critical load for the normal functioning of the ecosystem. In dozens of cities, lead concentrations in the air and soil exceed the values corresponding to the maximum permissible concentrations.
The highest level of air pollution with lead, exceeding the maximum permissible concentration, was observed in the cities of Komsomolsk-on-Amur, Tobolsk, Tyumen, Karabash, Vladimir, Vladivostok.
The maximum loads of lead deposition, leading to the degradation of terrestrial ecosystems, are observed in the Moscow, Vladimir, Nizhny Novgorod, Ryazan, Tula, Rostov and Leningrad regions.
Stationary sources are responsible for the discharge of more than 50 tons of lead in the form of various compounds into water bodies. At the same time, 7 battery factories discharge 35 tons of lead annually through the sewer system. An analysis of the distribution of lead discharges into water bodies in Russia shows that the Leningrad, Yaroslavl, Perm, Samara, Penza and Oryol regions are leaders in this type of load.
The country needs urgent measures to reduce lead pollution, but for now Russia's economic crisis is overshadowing environmental problems. In a long-running industrial depression, Russia lacks the means to clean up past pollution, but if the economy begins to recover and factories return to work, pollution could only worsen.
10 most polluted cities of the former USSR
(Metals are listed in descending order of priority level for a given city)
4. Soil hygiene. Waste disposal.
The soil in cities and other populated areas and their surroundings has long been different from natural, biologically valuable soil, which plays an important role in maintaining ecological balance. The soil in cities is subject to the same harmful effects as the urban air and hydrosphere, so significant degradation occurs everywhere. Soil hygiene is not given enough attention, although its importance as one of the main components of the biosphere (air, water, soil) and a biological environmental factor is even more significant than water, since the quantity of the latter (primarily the quality of groundwater) is determined by the condition of the soil, and it is impossible to separate these factors from each other. The soil has the ability of biological self-purification: in the soil, the breakdown of waste that enters it and its mineralization occurs; Ultimately, the soil compensates for the lost minerals at their expense.
If, as a result of overloading the soil, any of the components of its mineralizing ability is lost, this will inevitably lead to a disruption of the self-purification mechanism and to complete degradation of the soil. And, on the contrary, creating optimal conditions for self-purification of the soil helps maintain ecological balance and conditions for the existence of all living organisms, including humans.
Therefore, the problem of neutralizing waste that has harmful biological effects is not limited to the issue of their removal; it is a more complex hygienic problem, since soil is the link between water, air and humans.
4.1.
The role of soil in metabolism
The biological relationship between soil and humans is carried out mainly through metabolism. The soil is, as it were, a supplier of minerals necessary for the metabolic cycle, for the growth of plants consumed by humans and herbivores, which are in turn eaten by humans and carnivores. Thus, the soil provides food for many representatives of the plant and animal world.
Consequently, the deterioration of soil quality, a decrease in its biological value, and its ability to self-purify cause a biological chain reaction, which, in the case of prolonged harmful effects, can lead to a variety of health disorders among the population. Moreover, if mineralization processes are slowed down, nitrates, nitrogen, phosphorus, potassium, etc. formed during the breakdown of substances can enter groundwater used for drinking purposes and cause serious diseases (for example, nitrates can cause methemoglobinemia, primarily in infants).
Consumption of water from iodine-poor soil can cause endemic goiter, etc.
4.2.
Ecological relationship between soil and water and liquid waste (wastewater)
Man extracts from the soil the water necessary to maintain metabolic processes and life itself. Water quality depends on soil conditions; it always reflects the biological state of a given soil.
This especially applies to groundwater, the biological value of which is significantly determined by the properties of soil and soil, the latter’s ability to self-purify, its filtration capacity, the composition of its macroflora, microfauna, etc.
The direct influence of soil on surface waters is less significant; it is associated mainly with precipitation. For example, after heavy rains, various pollutants are washed from the soil into open bodies of water (rivers, lakes), including artificial fertilizers (nitrogen, phosphate), pesticides, herbicides; in areas of karst and fractured deposits, pollutants can penetrate through cracks into deep-lying The groundwater.
Inadequate wastewater treatment can also cause harmful biological effects on the soil and ultimately lead to soil degradation. Therefore, soil protection in populated areas is one of the main requirements for protecting the environment as a whole.
4.3.
Limits of soil load with solid waste (household and street garbage, industrial waste, dry sludge remaining after sedimentation of wastewater, radioactive substances, etc.)
The problem is compounded by the fact that, as a result of the generation of increasing amounts of solid waste in cities, the soil in their surroundings is subject to increasingly significant stress. The properties and composition of the soil are deteriorating at an increasingly rapid pace.
Of the 64.3 million tons of paper produced in the United States, 49.1 million tons end up in waste (of this amount, 26 million tons are “supplied” by households, and 23.1 million tons are supplied by retail chains).
In connection with the above, the removal and final neutralization of solid waste represents a very significant, more difficult to implement hygienic problem in the conditions of increasing urbanization.
The final neutralization of solid waste in contaminated soil seems possible. However, due to the constantly deteriorating ability of urban soil to self-purify, final neutralization of waste buried in the ground is impossible.
A person could successfully use the biochemical processes occurring in the soil, its neutralizing and disinfecting ability to neutralize solid waste, but urban soil, as a result of centuries of human habitation and activity in cities, has long become unsuitable for this purpose.
The mechanisms of self-purification and mineralization occurring in the soil, the role of the bacteria and enzymes involved in them, as well as intermediate and final products of the decomposition of substances are well known. Currently, research is aimed at identifying the factors that ensure the biological balance of natural soil, as well as at clarifying the question of what amount of solid waste (and what its composition) can lead to disruption of the biological balance of the soil.
Amount of household waste (garbage) per inhabitant of some major cities of the world
It should be noted that the hygienic condition of soil in cities quickly deteriorates as a result of its overload, although the ability of the soil to self-purify is the main hygienic requirement for maintaining biological balance. The soil in cities is no longer able to cope with its task without human help. The only way out of this situation position - full neutralization and destruction of waste in accordance with hygienic requirements.
Therefore, the construction of public utilities should be aimed at preserving the natural ability of the soil to self-purify, and if this ability has already become unsatisfactory, then it must be restored artificially.
The most unfavorable is the toxic effect of industrial waste, both liquid and solid. An increasing amount of such waste is entering the soil, which it is not able to cope with. For example, soil contamination with arsenic has been established in the vicinity of superphosphate production plants (within a radius of 3 km). As is known, some pesticides, such as organochlorine compounds that enter the soil, do not decompose for a long time.
The situation is similar with some synthetic packaging materials (polyvinyl chloride, polyethylene, etc.).
Some toxic compounds enter groundwater sooner or later, as a result of which not only the biological balance of the soil is disrupted, but the quality of groundwater also deteriorates to such an extent that it can no longer be used as drinking water.
Percentage of the number of main synthetic materials contained in household waste (garbage)
*
Together with waste of other heat-hardening plastics.
The problem of waste has increased these days also because part of the waste, mainly human and animal feces, is used to fertilize agricultural land [feces contain a significant amount of nitrogen - 0.4-0.5%, phosphorus (P203) - 0.2-0 .6%, potassium (K?0) -0.5-1.5%, carbon -5-15%]. This city problem has spread to the city's surrounding areas.
4.4.
The role of soil in the spread of various diseases
Soil plays a certain role in the spread of infectious diseases. This was reported back in the last century by Petterkoffer (1882) and Fodor (1875), who mainly highlighted the role of soil in the spread of intestinal diseases: cholera, typhoid fever, dysentery, etc. They also drew attention to the fact that some bacteria and viruses remain viable and virulent in the soil for months. Subsequently, a number of authors confirmed their observations, especially in relation to urban soil. For example, the causative agent of cholera remains viable and pathogenic in groundwater from 20 to 200 days, the causative agent of typhoid fever in feces - from 30 to 100 days, and the causative agent of paratyphoid fever - from 30 to 60 days. (From the point of view of the spread of infectious diseases, urban soil poses a much greater danger than field soil fertilized with manure.)
To determine the degree of soil contamination, a number of authors use the determination of bacterial count (Escherichia coli), as in determining water quality. Other authors consider it advisable to determine, in addition, the number of thermophilic bacteria taking part in the mineralization process.
The spread of infectious diseases through soil is greatly facilitated by irrigation of land with wastewater. At the same time, the mineralization properties of the soil deteriorate. Therefore, irrigation with wastewater should be carried out under constant strict sanitary supervision and only outside the urban area.
4.5.
Harmful effects of the main types of pollutants (solid and liquid waste) leading to soil degradation
4.5.1.
Neutralization of liquid waste in soil
In a number settlements where there is no sewage system, some waste, including manure, is neutralized in the soil.
As you know, this is the simplest method of neutralization. However, it is only permissible if we are dealing with biologically complete soil that has retained the ability to self-purify, which is not typical for urban soils. If the soil no longer possesses these qualities, then in order to protect it from further degradation, there is a need for complex technical structures for the neutralization of liquid waste.
In some places, waste is neutralized in compost pits. Technically, this solution is difficult task. In addition, liquids can penetrate the soil over fairly long distances. The task is further complicated by the fact that urban wastewater contains an increasing amount of toxic industrial waste, which worsens the mineralization properties of the soil to an even greater extent than human and animal feces. Therefore in compost pits It is permissible to discharge only wastewater that has been pre-sedimented. Otherwise, the filtration capacity of the soil is impaired, then the soil loses its other protective properties, pores gradually become clogged, etc.
The use of human feces to irrigate agricultural fields represents a second method of neutralizing liquid waste. This method poses a double hygienic danger: firstly, it can lead to soil overload; secondly, this waste can become a serious source of infection. Therefore, feces must first be disinfected and subjected to appropriate treatment and only then used as fertilizer. Here two opposing points of view collide. According to hygienic requirements, feces are subject to almost complete destruction, and from the point of view of the national economy they represent a valuable fertilizer. Fresh feces cannot be used to water gardens and fields without first disinfecting them. If you still have to use fresh feces, then they require such a degree of neutralization that they no longer represent almost any value as a fertilizer.
Feces can be used as fertilizer only in specially designated areas - with constant sanitary and hygienic control, especially over the state of groundwater, quantity, flies, etc.
The requirements for the removal and soil neutralization of animal feces are, in principle, no different from the requirements for the neutralization of human feces.
Until recently, manure represented in agriculture a significant source of valuable nutrients necessary to increase soil fertility. However, in recent years, manure has lost its importance, partly due to the mechanization of agriculture, partly due to the increasing use of artificial fertilizers.
In the absence of appropriate treatment and neutralization, manure is also dangerous, just like unneutralized human feces. Therefore, before being taken out to the fields, manure is allowed to ripen so that during this time the necessary biothermal processes can occur in it (at a temperature of 60-70°C). After this, the manure is considered “mature” and freed from most of the pathogens it contains (bacteria, worm eggs, etc.).
It must be remembered that manure storage facilities can provide ideal breeding grounds for flies that contribute to the spread of various intestinal infections. It should be noted that flies most readily choose pig manure for breeding, then horse manure, sheep manure, and lastly cow manure. Before transporting manure to fields, it must be treated with insecticides.
continuation
--PAGE_BREAK--
Heavy metals in soil
Recently, due to the rapid development of industry, there has been a significant increase in the level of heavy metals in the environment. The term "heavy metals" applies to metals either with a density exceeding 5 g/cm 3 or with an atomic number greater than 20. Although, there is another point of view, according to which heavy metals include over 40 chemical elements with atomic masses exceeding 50 at. units Among chemical elements, heavy metals are the most toxic and are second only to pesticides in their level of danger. At the same time, the following chemical elements are considered toxic: Co, Ni, Cu, Zn, Sn, As, Se, Te, Rb, Ag, Cd, Au, Hg, Pb, Sb, Bi, Pt.
The phytotoxicity of heavy metals depends on their chemical properties: valence, ionic radius and complexability. In most cases, elements are arranged in the order of toxicity: Cu > Ni > Cd > Zn > Pb > Hg > Fe > Mo > Mn. However, this series may vary somewhat due to unequal precipitation of elements by the soil and transfer to a state inaccessible to plants, growing conditions, and the physiological and genetic characteristics of the plants themselves. The transformation and migration of heavy metals occurs under the direct and indirect influence of the complexation reaction. When assessing environmental pollution, it is necessary to take into account the properties of the soil and, first of all, the granulometric composition, humus content and buffering capacity. Buffer capacity refers to the ability of soils to maintain the concentration of metals in the soil solution at a constant level.
In soils, heavy metals are present in two phases - solid and in soil solution. The form of existence of metals is determined by the reaction of the environment, the chemical and material composition of the soil solution and, first of all, the content of organic substances. Complexing elements that pollute the soil are concentrated mainly in its upper 10 cm layer. However, when low-buffer soil is acidified, a significant proportion of metals from the exchange-absorbed state passes into the soil solution. Cadmium, copper, nickel, and cobalt have a strong migration ability in an acidic environment. A decrease in pH by 1.8-2 units leads to an increase in the mobility of zinc by 3.8-5.4, cadmium by 4-8, copper by 2-3 times.
Table 1 Maximum permissible concentration (MAC) standards, background contents of chemical elements in soils (mg/kg)
| Element | Hazard Class | MPC | UEC by soil groups | Background content | |||
| Gross content | Extractable with ammonium acetate buffer (pH=4.8) | Sandy, sandy loam | Loamy, clayey | ||||
| pH x l< 5,5 | pH x l > 5.5 | ||||||
| Pb | 1 | 32 | 6 | 32 | 65 | 130 | 26 |
| Zn | 1 | - | 23 | 55 | 110 | 220 | 50 |
| Cd | 1 | - | - | 0,5 | 1 | 2 | 0,3 |
| Cu | 2 | - | 3 | 33 | 66 | 132 | 27 |
| Ni | 2 | - | 4 | 20 | 40 | 80 | 20 |
| Co | 2 | - | 5 | - | - | - | 7,2 |
Thus, when heavy metals enter the soil, they quickly interact with organic ligands to form complex compounds. So, at low concentrations in soil (20-30 mg/kg), approximately 30% of lead is in the form of complexes with organic matter. The proportion of complex lead compounds increases with increasing concentration up to 400 mg/g, and then decreases. Metals are also sorbed (exchangeably or nonexchangeably) by sediments of iron and manganese hydroxides, clay minerals, and soil organic matter. Metals available to plants and capable of leaching are found in the soil solution in the form of free ions, complexes and chelates.
The absorption of HMs by soil largely depends on the reaction of the environment and on which anions predominate in the soil solution. In an acidic environment, copper, lead and zinc are more sorbed, and in an alkaline environment, cadmium and cobalt are intensively absorbed. Copper preferentially binds to organic ligands and iron hydroxides.
Table 2 Mobility of microelements in various soils depending on the pH of the soil solution
Soil and climatic factors often determine the direction and speed of migration and transformation of HMs in the soil. Thus, the conditions of the soil and water regimes of the forest-steppe zone contribute to intensive vertical migration of HM along the soil profile, including the possible transfer of metals with water flow along cracks, root passages, etc.
Nickel (Ni) is an element of Group VIII of the periodic table with an atomic mass of 58.71. Nickel, along with Mn, Fe, Co and Cu, belongs to the so-called transition metals, the compounds of which have high biological activity. Due to the structural features of electronic orbitals, the above metals, including nickel, have a pronounced ability to form complexes. Nickel is capable of forming stable complexes, for example, with cysteine and citrate, as well as with many organic and inorganic ligands. The geochemical composition of source rocks largely determines the nickel content in soils. The greatest amount of nickel is contained in soils formed from basic and ultrabasic rocks. According to some authors, the boundaries of excess and toxic levels of nickel for most species vary from 10 to 100 mg/kg. The bulk of nickel is immovably fixed in the soil, and very weak migration in the colloidal state and in the composition of mechanical suspensions does not affect their distribution along the vertical profile and is quite uniform.
Lead (Pb). The chemistry of lead in the soil is determined by the delicate balance of oppositely directed processes: sorption-desorption, dissolution-transition to the solid state. Lead released into the soil is included in a cycle of physical, chemical and physicochemical transformations. At first, the processes of mechanical movement (lead particles move along the surface and through cracks in the soil) and convective diffusion dominate. Then, as solid-phase lead compounds dissolve, more complex physical and chemical processes come into play (in particular, processes of ion diffusion), accompanied by the transformation of lead compounds arriving with dust.
It has been established that lead migrates both vertically and horizontally, with the second process prevailing over the first. Over 3 years of observations in a mixed-grass meadow, lead dust applied locally to the soil surface moved horizontally by 25-35 cm, and the depth of its penetration into the soil thickness was 10-15 cm. Biological factors play an important role in the migration of lead: plant roots absorb ions metals; during the growing season they move through the soil; When plants die and decompose, lead is released into the surrounding soil mass.
It is known that soil has the ability to bind (sorb) technogenic lead entering it. Sorption is believed to include several processes: complete exchange with cations of the soil absorbing complex (nonspecific adsorption) and a series of reactions of lead complexation with donors of soil components (specific adsorption). In soil, lead is associated mainly with organic matter, as well as with clay minerals, manganese oxides, and iron and aluminum hydroxides. By binding lead, humus prevents its migration into adjacent environments and limits its entry into plants. Of the clay minerals, illites are characterized by a tendency to sorption of lead. An increase in soil pH during liming leads to an even greater binding of lead in the soil due to the formation of sparingly soluble compounds (hydroxides, carbonates, etc.).
Lead, present in the soil in mobile forms, is fixed by soil components over time and becomes inaccessible to plants. According to domestic researchers, lead is most firmly fixed in chernozem and peat-silt soils.
Cadmium (Cd) The peculiarity of cadmium, which distinguishes it from other HMs, is that in the soil solution it is present mainly in the form of cations (Cd 2+), although in soil with a neutral reaction environment it can form sparingly soluble complexes with sulfates and phosphates or hydroxides.
According to available data, the concentration of cadmium in soil solutions of background soils ranges from 0.2 to 6 μg/l. In areas of soil pollution it increases to 300-400 µg/l.
It is known that cadmium in soils is very mobile, i.e. is capable of moving in large quantities from the solid phase to the liquid phase and back (which makes it difficult to predict its entry into the plant). The mechanisms that regulate the concentration of cadmium in the soil solution are determined by sorption processes (by sorption we mean adsorption itself, precipitation and complexation). Cadmium is absorbed by soil in smaller quantities than other HMs. To characterize the mobility of heavy metals in soil, the ratio of metal concentrations in the solid phase to that in the equilibrium solution is used. High values of this ratio indicate that heavy metals are retained in the solid phase due to the sorption reaction, while low values indicate that metals are in solution, from where they can migrate to other media or enter into various reactions (geochemical or biological). It is known that the leading process in the binding of cadmium is adsorption by clays. Research in recent years has also shown the important role of hydroxyl groups, iron oxides and organic matter in this process. When the level of pollution is low and the reaction of the environment is neutral, cadmium is adsorbed mainly by iron oxides. And in an acidic environment (pH=5), organic matter begins to act as a powerful adsorbent. At lower pH values (pH=4), adsorption functions shift almost exclusively to organic matter. Mineral components cease to play any role in these processes.
It is known that cadmium is not only sorbed by the soil surface, but is also fixed due to precipitation, coagulation, and interpacket absorption by clay minerals. It diffuses inside soil particles through micropores and other ways.
Cadmium is fixed in different ways in soils different types. So far, little is known about the competitive relationships of cadmium with other metals in sorption processes in the soil-absorbing complex. According to research by specialists from the Technical University of Copenhagen (Denmark), in the presence of nickel, cobalt and zinc, the absorption of cadmium by the soil was suppressed. Other studies have shown that the processes of cadmium sorption by soil are damped in the presence of chlorine ions. Saturation of soil with Ca 2+ ions led to an increase in cadmium sorption. Many bonds of cadmium with soil components turn out to be fragile; under certain conditions (for example, an acidic reaction of the environment), it is released and goes back into solution.
The role of microorganisms in the process of dissolution of cadmium and its transition to a mobile state has been revealed. As a result of their vital activity, either water-soluble metal complexes are formed, or physicochemical conditions are created that are favorable for the transition of cadmium from the solid phase to the liquid phase.
The processes occurring with cadmium in the soil (sorption-desorption, transition into solution, etc.) are interconnected and interdependent; the supply of this metal to plants depends on their direction, intensity and depth. It is known that the amount of cadmium sorption by soil depends on the pH value: the higher the soil pH, the more cadmium it sorbs. Thus, according to available data, in the pH range from 4 to 7.7, with an increase in pH by one unit, the sorption capacity of soils with respect to cadmium increased approximately threefold.
Zinc (Zn). Zinc deficiency can manifest itself both on acidic, highly podzolized light soils, and on carbonate soils, poor in zinc, and on highly humus-rich soils. The manifestation of zinc deficiency is enhanced by the use of high doses of phosphorus fertilizers and strong plowing of the subsoil to the arable horizon.
The highest gross zinc content is in tundra (53-76 mg/kg) and chernozem (24-90 mg/kg) soils, the lowest in soddy-podzolic soils (20-67 mg/kg). Zinc deficiency most often occurs on neutral and slightly alkaline carbonate soils. In acidic soils, zinc is more mobile and available to plants.
Zinc in soil is present in ionic form, where it is adsorbed by a cation exchange mechanism in an acidic environment or as a result of chemisorption in an alkaline environment. The most mobile ion is Zn 2+. The mobility of zinc in soil is mainly affected by pH and the content of clay minerals. At pH<6 подвижность Zn 2+ возрастает, что приводит к его выщелачиванию. Попадая в межпакетные пространства кристаллической решетки монтмориллонита, ионы цинка теряют свою подвижность. Кроме того, цинк образует устойчивые формы с органическим веществом почвы, поэтому он накапливается в основном в горизонтах почв с высоким содержанием гумуса и в торфе.
Heavy metals in plants
According to A.P. Vinogradov (1952), all chemical elements participate to one degree or another in the life of plants, and if many of them are considered physiologically significant, it is only because there is no evidence for this yet. Entering the plant in small quantities and becoming an integral part or activator of enzymes, microelements perform service functions in metabolic processes. When unusually high concentrations of elements enter the environment, they become toxic to plants. The penetration of heavy metals into plant tissue in excess amounts leads to disruption of the normal functioning of their organs, and this disruption is stronger, the greater the excess of toxicants. Productivity drops as a result. The toxic effect of HMs manifests itself from the early stages of plant development, but to varying degrees on different soils and for different crops.
The absorption of chemical elements by plants is an active process. Passive diffusion accounts for only 2-3% of the total mass of absorbed mineral components. When the content of metals in the soil is at the background level, active absorption of ions occurs, and if we take into account the low mobility of these elements in soils, then their absorption should be preceded by the mobilization of tightly bound metals. When the content of heavy metals in the root layer is in quantities significantly exceeding the maximum concentrations at which the metal can be fixed using the internal resources of the soil, such quantities of metals enter the roots that the membranes can no longer retain them. As a result, the supply of ions or compounds of elements is no longer regulated by cellular mechanisms. On acidic soils there is a more intense accumulation of HMs than on soils with a neutral or close to neutral reaction environment. A measure of the actual participation of HM ions in chemical reactions is their activity. The toxic effect of high concentrations of heavy metals on plants can manifest itself in disruption of the supply and distribution of other chemical elements. The nature of the interaction of heavy metals with other elements varies depending on their concentrations. Migration and entry into the plant occurs in the form of complex compounds.
During the initial period of environmental contamination with heavy metals, due to the buffer properties of the soil, leading to the inactivation of toxicants, plants will experience virtually no adverse effects. However, the protective functions of soil are not unlimited. As the level of heavy metal pollution increases, their inactivation becomes incomplete and the flow of ions attacks the roots. The plant is able to convert some of the ions into a less active state even before they penetrate into the plant root system. This is, for example, chelation using root secretions or adsorption on the outer surface of roots with the formation of complex compounds. In addition, as vegetation experiments with obviously toxic doses of zinc, nickel, cadmium, cobalt, copper, and lead have shown, the roots are located in layers not contaminated with HM soils and in these cases there are no symptoms of phototoxicity.
Despite the protective functions of the root system, heavy metals enter the root under polluted conditions. In this case, protection mechanisms come into play, thanks to which a specific distribution of HMs occurs among plant organs, making it possible to protect their growth and development as completely as possible. Moreover, the content of, for example, heavy metals in the tissues of roots and seeds in highly polluted environments can vary by 500-600 times, which indicates the great protective capabilities of this underground plant organ.
Excess of chemical elements causes toxicosis in plants. As the concentration of heavy metals increases, plant growth is first retarded, then leaf chlorosis occurs, which is replaced by necrosis, and, finally, the root system is damaged. The toxic effect of HM can manifest itself directly and indirectly. The direct effect of excess heavy metals in plant cells is due to complexation reactions, which result in enzyme blocking or protein precipitation. Deactivation of enzymatic systems occurs as a result of the replacement of the enzyme metal with a pollutant metal. When the toxicant content is critical, the catalytic ability of the enzyme is significantly reduced or completely blocked.
Plants are hyperaccumulators of heavy metals
A.P. Vinogradov (1952) identified plants that are capable of concentrating elements. He pointed to two types of plants - concentrators: 1) plants that concentrate elements on a mass scale; 2) plants with selective (species) concentration. Plants of the first type are enriched with chemical elements if the latter are contained in the soil in increased quantities. Concentration in this case is caused by an environmental factor. Plants of the second type are characterized by a constantly high amount of one or another chemical element, regardless of its content in the environment. It is determined by a genetically fixed need.
Considering the mechanism of absorption of heavy metals from soil into plants, we can talk about barrier (non-concentrating) and barrier-free (concentrating) types of accumulation of elements. Barrier accumulation is typical for most higher plants and is not typical for bryophytes and lichens. Thus, in the work of M.A. Toikka and L.N. Potekhina (1980), sphagnum (2.66 mg/kg) was named as a plant-concentrator of cobalt; copper (10.0 mg/kg) - birch, drupe, lily of the valley; manganese (1100 mg/kg) - blueberries. Lepp et al. (1987) found high concentrations of cadmium in the sporophores of the fungus Amanita muscaria growing in birch forests. In the sporophores of the fungus, the cadmium content was 29.9 mg/kg of dry weight, and in the soil on which they grew - 0.4 mg/kg. There is an opinion that plants that are concentrators of cobalt are also highly tolerant to nickel and are able to accumulate it in large quantities. These include, in particular, plants of the families Boraginaceae, Brassicaceae, Myrtaceae, Fabaceae, Caryophyllaceae. Nickel concentrators and superconcentrators have also been found among medicinal plants. Superconcentrators include melon tree, belladonna belladonna, yellow poppy, motherwort cordial, passionflower and Thermopsis lanceolata. The type of accumulation of chemical elements found in high concentrations in the nutrient medium depends on the phases of plant growth. Barrier-free accumulation is characteristic of the seedling phase, when plants do not differentiate the above-ground parts into various organs, and in the final phases of the growing season - after ripening, as well as during the period of winter dormancy, when barrier-free accumulation may be accompanied by the release of excess amounts of chemical elements in the solid phase (Kovalevsky, 1991).
Hyperaccumulating plants are found in the families Brassicaceae, Euphorbiaceae, Asteraceae, Lamiaceae and Scrophulariaceae (Baker 1995). The most famous and studied among them is Brassica juncea (Indian mustard), a plant that develops large biomass and is capable of accumulating Pb, Cr (VI), Cd, Cu, Ni, Zn, 90Sr, B and Se (Nanda Kumar et al. 1995 ; Salt et al. 1995; Raskin et al. 1994). Of the different plant species tested, B. juncea had the most pronounced ability to transport lead aboveground, accumulating more than 1.8% of this element in aboveground organs (based on dry weight). With the exception of sunflower (Helianthus annuus) and tobacco (Nicotiana tabacum), other non-Brassicaceae plant species had a biological uptake coefficient of less than 1.
According to the classification of plants according to their response to the presence of heavy metals in their growing environment, used by many foreign authors, plants have three main strategies for growth on soils contaminated with metals:
Metal excluders. Such plants maintain a constant low concentration of metal despite wide variations in its concentrations in the soil, retaining mainly the metal in the roots. Exclusive plants are capable of changing membrane permeability and metal-binding capacity of cell walls or releasing large amounts of chelating substances.
Metal indicators. These include plant species that actively accumulate metal in above-ground parts and generally reflect the level of metal content in the soil. They tolerate the existing level of metal concentration due to the formation of extracellular metal-binding compounds (chelators), or change the nature of metal compartmentation by storing it in metal-insensitive areas. Metal-accumulating plant species. Plants belonging to this group can accumulate the metal in above-ground biomass in concentrations much higher than those in the soil. Baker and Brooks defined metal hyperaccumulators as plants containing more than 0.1%, i.e. more than 1000 mg/g copper, cadmium, chromium, lead, nickel, cobalt or 1% (more than 10,000 mg/g) zinc and manganese in dry weight. For rare metals, this value is more than 0.01% in terms of dry weight. Researchers identify hyperaccumulating species by collecting plants in areas where soils contain metals in concentrations above background levels, as is the case in contaminated areas or where ore bodies are exposed. The phenomenon of hyperaccumulation raises many questions for researchers. For example, what is the significance of the accumulation of metal in highly toxic concentrations for plants? A definitive answer to this question has not yet been received, but there are several main hypotheses. It is assumed that such plants have an enhanced ion uptake system (the "unintentional" uptake hypothesis) to perform certain physiological functions that have not yet been studied. It is also believed that hyperaccumulation is one of the types of plant tolerance to high metal content in the growing environment.
Heavy metals (HM) include more than 40 chemical elements of D.I. Mendeleev’s periodic table, the mass of atoms of which is over 50 atomic mass units (amu). These are Pb, Zn, Cd, Hg, Cu, Mo, Mn, Ni, Sn, Co, etc.
The established concept of “heavy metals” is not strict, since HMs often include non-metal elements, for example As, Se, and sometimes even F, Be and other elements whose atomic mass is less than 50 amu.
There are many trace elements among HMs that are biologically important for living organisms. They are necessary and indispensable components of biocatalysts and bioregulators of the most important physiological processes. However, the excess content of heavy metals in various objects of the biosphere has a depressing and even toxic effect on living organisms.
Sources of heavy metals entering the soil are divided into natural (weathering of rocks and minerals, erosion processes, volcanic activity) and technogenic (extraction and processing of minerals, fuel combustion, influence of vehicles, agriculture, etc.) Agricultural lands, in addition to pollution through atmosphere, HMs are also polluted specifically, through the use of pesticides, mineral and organic fertilizers, liming, and the use of wastewater. Recently, scientists have paid special attention to urban soils. The latter are experiencing significant man-made pressure, part of which is HM pollution.
In table 3.14 and 3.15 present the distribution of HM in various objects of the biosphere and the sources of HM entry into the environment.
Table 3.14
| Element | Soils | Fresh waters | sea waters | Plants | Animals (in muscle tissue) |
| Mn | 1000 | 0,008 | 0,0002 | 0,3-1000 | 0,2-2,3 |
| Zn | 90 (1-900) | 0,015 | 0,0049 | 1,4-600 | 240 |
| Cu | 30 (2-250) | 0,003 | 0,00025 | 4-25 | 10 |
| Co | 8 (0,05-65) | 0,0002 | 0,00002 | 0,01-4,6 | 0,005-1 |
| Pb | 35 (2-300) | 0,003 | 0,00003 | 0,2-20 | 0,23-3,3 |
| Cd | 0,35 (0,01-2) | 0,0001 | - | 0,05-0,9 | 0,14-3,2 |
| Hg | 0,06 | 0,0001 | 0,00003 | 0,005-0,02 | 0,02-0,7 |
| As | 6 | 0,0005 | 0,0037 | 0,02-7 | 0,007-0,09 |
| Se | 0,4 (0,01-12) | 0,0002 | 00,0002 | 0,001-0,5 | 0,42-1,9 |
| F | 200 | 0,1 | 1,3 | 0,02-24 | 0,05 |
| B | 20 (2-270) | 0,15 | 4,44 | 8-200 | 0,33-1 |
| Mo | 1,2 (0,1-40) | 0,0005 | 0,01 | 0,03-5 | 0,02-0,07 |
| Cr | 70 (5-1500) | 0,001 | 0,0003 | 0,016-14 | 0,002-0,84 |
| Ni | 50 (2-750) | 0,0005 | 0,00058 | 0,02-4 | 1-2 |
Table 3.15
Sources of environmental pollution TM
End of table. 3.4
HMs reach the soil surface in various forms. These are oxides and various salts of metals, both soluble and practically insoluble in water (sulfides, sulfates, arsenites, etc.). In the emissions of ore processing enterprises and non-ferrous metallurgy enterprises - the main source of environmental pollution with heavy metals - the bulk of metals (70-90%) are in the form of oxides.
Once on the soil surface, HMs can either accumulate or dissipate, depending on the nature of the geochemical barriers inherent in a given area.
Most of the HMs arriving on the soil surface are fixed in the upper humus horizons. HMs are sorbed on the surface of soil particles, bind to soil organic matter, in particular in the form of elemental organic compounds, accumulate in iron hydroxides, form part of the crystal lattices of clay minerals, produce their own minerals as a result of isomorphic replacement, and are in a soluble state in soil moisture and gaseous state in the soil air, are an integral part of the soil biota.
The degree of mobility of heavy metals depends on the geochemical situation and the level of technogenic impact. The heavy particle size distribution and high content of organic matter lead to the binding of HMs in the soil. An increase in pH values increases the sorption of cation-forming metals (copper, zinc, nickel, mercury, lead, etc.) and increases the mobility of anion-forming metals (molybdenum, chromium, vanadium, etc.). Increasing oxidative conditions increases the migration ability of metals. As a result, according to their ability to bind the majority of HMs, soils form the following series: gray soil > chernozem > soddy-podzolic soil.
The duration of residence of polluting components in the soil is much longer than in other parts of the biosphere, and soil contamination, especially with heavy metals, is almost eternal. Metals accumulate in the soil and are slowly removed through leaching, plant consumption, erosion and deflation (Kabata-Pendias and Pendias, 1989). The period of half-removal (or removal of half of the initial concentration) of HM varies greatly for different elements, but is quite long periods of time: for Zn - from 70 to 510 years; for Cd - from 13 to 110 years; for Cu - from 310 to 1500 years and for Pb - 2 - from 740 to 5900 years (Sadovskaya, 1994).
Soil contamination with heavy metals has two negative aspects. Firstly, entering through food chains from the soil into plants, and from there into the body of animals and humans, heavy metals cause serious diseases in them - an increase in the incidence of the population and a reduction in life expectancy, as well as a decrease in the quantity and quality of harvests of agricultural plants and livestock products.
Secondly, accumulating in large quantities in the soil, HMs are capable of changing many of its properties. First of all, the changes affect the biological properties of the soil: the total number of microorganisms decreases, their species composition (diversity) narrows, the structure of microbial communities changes, the intensity of the main microbiological processes and activity of soil enzymes, etc. Severe contamination with heavy metals leads to changes in more conservative characteristics of the soil, such as humus state, structure, pH of the environment, etc. The result of this is partial, and in some cases, complete loss of soil fertility.
In nature, there are areas with insufficient or excessive content of HMs in soils. The abnormal content of heavy metals in soils is due to two groups of reasons: biogeochemical characteristics of ecosystems and the influence of technogenic flows of matter. In the first case, areas where the concentration of chemical elements is higher or lower than the optimal level for living organisms are called natural geochemical anomalies, or biogeochemical provinces. Here, the anomalous content of elements is due to natural causes - the characteristics of soil-forming rocks, the soil-forming process, and the presence of ore anomalies. In the second case, the territories are called man-made geochemical anomalies. Depending on the scale, they are divided into global, regional and local.
Soil, unlike other components of the natural environment, not only geochemically accumulates pollution components, but also acts as a natural buffer that controls the transfer of chemical elements and compounds into the atmosphere, hydrosphere and living matter.
Various plants, animals and humans require a certain composition of soil and water for their life. In places of geochemical anomalies, aggravated transmission of deviations from the norm in mineral composition occurs throughout the food chain.
As a result of disturbances in mineral nutrition, changes in the species composition of phyto-, zoo- and microbiocenoses, diseases of wild plant forms, a decrease in the quantity and quality of crops of agricultural plants and livestock products, an increase in morbidity in the population and a decrease in life expectancy are observed (Table 3.15). The mechanism of toxic action of HM is presented in Table. 3.16.
Table 3.15
Physiological disorders in plants with excess and deficiency of HM content in them (according to Kovalevsky, Andrianova, 1970; Kabata-pendias,
Pendas, 1989)
| Element | Physiological disorders | |
| in case of shortage | in case of excess | |
| Cu | Chlorosis, wilt, melanism, white curled crowns, weakened panicle formation, impaired lignification, dry tops of trees | Dark green leaves, as in Fe-induced chlorosis; thick, short or barbed wire-like roots, inhibition of shoot formation |
| Zn | Interveinal chlorosis (mainly in monocots), growth arrest, rosette leaves of trees, purple-red dots on leaves | Chlorosis and necrosis of leaf tips, interveinal chlorosis of young leaves, stunted growth of the plant as a whole, damaged roots that look like barbed wire |
| Cd | - | Brown leaf edges, chlorosis, reddish veins and petioles, curled leaves and brown underdeveloped roots |
| Hg | - | Some inhibition of sprouts and roots, chlorosis of leaves and brown spots on them |
| Pb | - | Reduced photosynthesis rate, dark green leaves, curling of old leaves, stunted foliage, brown short roots |
Table 3.16
Mechanism of action of HM toxicity (according to Torshin et al., 1990)
| Element | Action |
| Cu, Zn, Cd, Hg, Pb | Effect on membrane permeability, reaction with SH - groups of cysteine and methionine |
| Pb | Changing the three-dimensional structure of proteins |
| Cu, Zn, Hg, Ni | Formation of complexes with phospholipids |
| Ni | Formation of complexes with albumin |
| Enzyme inhibition: | |
| Hg2+ | alkaline phosphatase, gluco-6-phosphatase, lactate dehydrogenase |
| Cd2+ | adenosine triphosphatases, alcohol dehydrogenases, amylases, carbonic anhydrases, carboxypeptidases (pentidases), glutamate oxaloacetate transaminases |
| Pb2+ | acetylcholinesterase, alkaline phosphatase, ATPase |
| Ni2+ | carbonic anhydrase, cytochrome oxidase, benzopyrene hydroxylase |
Toxic effects of HM on biological systems primarily due to the fact that they easily bind to sulfhydryl groups of proteins (including enzymes), suppressing their synthesis and thereby disrupting metabolism in the body.
Living organisms have developed various mechanisms of resistance to HMs: from the reduction of HM ions into less toxic compounds to the activation of ion transport systems that effectively and specifically remove toxic ions from the cell into the external environment.
The most significant consequence of the impact of heavy metals on living organisms, which manifests itself at the biogeocenotic and biosphere levels of organization of living matter, is the blocking of the oxidation processes of organic matter. This leads to a decrease in the rate of its mineralization and accumulation in ecosystems. At the same time, an increase in the concentration of organic matter causes it to bind HM, which temporarily relieves the load on the ecosystem. A decrease in the rate of decomposition of organic matter due to a decrease in the number of organisms, their biomass and the intensity of vital activity is considered a passive response of ecosystems to HM pollution. Active resistance of organisms to anthropogenic loads manifests itself only during the lifetime accumulation of metals in bodies and skeletons. The most resistant species are responsible for this process.
The resistance of living organisms, primarily plants, to elevated concentrations of heavy metals and their ability to accumulate high concentrations of metals can pose a great danger to human health, since they allow the penetration of pollutants into food chains. Depending on the geochemical conditions of production, human food of both plant and animal origin can satisfy human needs for mineral elements, be deficient or contain an excess of them, becoming more toxic, causing diseases and even death (Table 3.17).
Table 3.17
Effect of HM on the human body (Kovalsky, 1974; Concise Medical Encyclopedia, 1989; Torshin et al., 1990; Impact on the body.., 1997; Handbook of toxicology.., 1999)
| Element | Physiological abnormalities | |
| in case of shortage | in case of excess | |
| Mn | Diseases of the skeletal system | Fever, pneumonia, damage to the central nervous system (manganese parkinsonism), endemic gout, circulatory disorders, gastrointestinal functions, infertility |
| Cu | Weakness, anemia, leukemia, diseases of the skeletal system, impaired coordination of movements | Occupational diseases, hepatitis, Wilson's disease. Affects kidneys, liver, brain, eyes |
| Zn | Decreased appetite, bone deformation, dwarfism, long healing of wounds and burns, poor vision, myopia | Decreased cancer resistance, anemia, inhibition of oxidative processes, dermatitis |
| Pb | - | Lead encephaloneuropathy, metabolic disorders, inhibition of enzymatic reactions, vitamin deficiency, anemia, multiple sclerosis. Part of the skeletal system instead of calcium |
| Cd | - | Gastrointestinal disorders, respiratory disorders, anemia, increased blood pressure, kidney damage, itai-itai disease, proteinuria, osteoporosis, mutagenic and carcinogenic effects |
| Hg | - | Lesions of the central nervous system and peripheral nerves, infantilism, reproductive dysfunction, stomatitis, disease Minamata, premature aging |
| Co | Endemic goiter | - |
| Ni | - | Dermatitis, hematopoietic disorder, carcinogenicity, embryotoxicosis, subacute myelo-optic neuropathy |
| Cr | - | Dermatitis, carcinogenicity |
| V | - | Diseases of the cardiovascular system |
Different HMs pose a threat to human health to varying degrees. The most dangerous are Hg, Cd, Pb (Table 3.18).
Table 3.18
Classes of pollutants according to their degree of danger (GOST 17.4.1.02-83)
The issue of regulating the content of heavy metals in soil is very complicated. Its solution should be based on the recognition of the multifunctionality of the soil. In the process of rationing, soil can be viewed from different positions: as a natural body; as a habitat and substrate for plants, animals and microorganisms; as an object and means of agricultural and industrial production; as a natural reservoir containing pathogenic microorganisms. Standardization of HM content in soil must be carried out on the basis of soil-ecological principles, which deny the possibility of finding uniform values for all soils.
There are two main approaches to the issue of remediation of soils contaminated with heavy metals. The first is aimed at clearing the soil of HM. Purification can be carried out by leaching, by extracting HMs from the soil with the help of plants, by removing the top contaminated layer of soil, etc. The second approach is based on fixing HMs in the soil, converting them into forms that are insoluble in water and inaccessible to living organisms. For this purpose, it is proposed to introduce organic matter, phosphorus mineral fertilizers, ion exchange resins, natural zeolites, brown coal, liming of the soil, etc. into the soil. However, any method of fixing HMs in the soil has its own validity period. Sooner or later, part of the HM will again begin to enter the soil solution, and from there into living organisms.
Thus, heavy metals include more than 40 chemical elements, the mass of atoms of which is over 50 a. eat. These are Pb, Zn, Cd, Hg, Cu, Mo, Mn, Ni, Sn, Co, etc. There are many trace elements among HMs, which are necessary and irreplaceable components of biocatalysts and bioregulators of the most important physiological processes. However, the excess content of heavy metals in various objects of the biosphere has a depressing and even toxic effect on living organisms.
Sources of heavy metals entering the soil are divided into natural (weathering of rocks and minerals, erosion processes, volcanic activity) and technogenic (mining and processing of minerals, fuel combustion, influence of motor transport, agriculture, etc.).
HMs reach the soil surface in various forms. These are oxides and various salts of metals, both soluble and practically insoluble in water.
The environmental consequences of soil contamination with heavy metals depend on the pollution parameters, geochemical conditions and soil stability. The pollution parameters include the nature of the metal, i.e. its chemical and toxic properties, the metal content in the soil, the form of the chemical compound, the period from the moment of pollution, etc. The resistance of soils to pollution depends on the particle size distribution, the content of organic matter, acidity alkaline and redox conditions, activity of microbiological and biochemical processes, etc.
The resistance of living organisms, primarily plants, to elevated concentrations of heavy metals and their ability to accumulate high concentrations of metals can pose a great danger to human health, since they allow the penetration of pollutants into food chains.
When regulating the content of heavy metals in soil, the multifunctionality of the soil should be taken into account. Soil can be considered as a natural body, as a habitat and substrate for plants, animals and microorganisms, as an object and means of agricultural and industrial production, as a natural reservoir containing pathogenic microorganisms, as part of the terrestrial biogeocenosis and the biosphere as a whole.
